Nicotinic acetylcholine receptor ligands, a patent review (2006-2011)
- PMID: 22098319
- PMCID: PMC3495178
- DOI: 10.1517/13543776.2011.637919
Nicotinic acetylcholine receptor ligands, a patent review (2006-2011)
Abstract
Introduction: Nicotinic acetylcholine receptors (nAChRs), pentameric ligand-gated cation channels, are potential targets for the development of therapeutics for a variety of disease states.
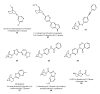
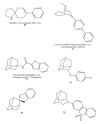
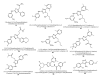
Areas covered: This article is reviewing recent advances in the development of small-molecule ligands for diverse nAChR subtypes and is a continuation of an earlier review in this journal.
Expert opinion: The development of nAChR ligands with preference for α4β2 or α7 subtypes for the treatment of central nervous system disorders are in the most advanced developmental stage. In addition, there is a fast growing interest to generate so-called PAMs, positive allosteric modulators, to influence the channels' functionalities.
Figures






Similar articles
-
Positive allosteric modulators as an approach to nicotinic acetylcholine receptor-targeted therapeutics: advantages and limitations.Biochem Pharmacol. 2011 Oct 15;82(8):915-30. doi: 10.1016/j.bcp.2011.05.001. Epub 2011 May 14. Biochem Pharmacol. 2011. PMID: 21575610 Free PMC article. Review.
-
Targeting α4β2 nicotinic acetylcholine receptors in central nervous system disorders: perspectives on positive allosteric modulation as a therapeutic approach.Basic Clin Pharmacol Toxicol. 2015 Mar;116(3):187-200. doi: 10.1111/bcpt.12361. Epub 2014 Dec 29. Basic Clin Pharmacol Toxicol. 2015. PMID: 25441336 Review.
-
Multiple interaction regions in the orthosteric ligand binding domain of the α7 neuronal nicotinic acetylcholine receptor.J Chem Inf Model. 2012 Nov 26;52(11):3064-73. doi: 10.1021/ci3001953. Epub 2012 Nov 6. J Chem Inf Model. 2012. PMID: 23092444
-
Therapeutic potential of novel selective drugs targeting nicotinic acetylcholine receptors.J Mol Neurosci. 2006;30(1-2):17-8. doi: 10.1385/JMN:30:1:17. J Mol Neurosci. 2006. PMID: 17192609
-
Nicotinic receptors: allosteric transitions and therapeutic targets in the nervous system.Nat Rev Drug Discov. 2009 Sep;8(9):733-50. doi: 10.1038/nrd2927. Nat Rev Drug Discov. 2009. PMID: 19721446 Review.
Cited by
-
Nicotinic Receptor Subunits Atlas in the Adult Human Lung.Int J Mol Sci. 2020 Oct 9;21(20):7446. doi: 10.3390/ijms21207446. Int J Mol Sci. 2020. PMID: 33050277 Free PMC article.
-
Cholinergic System and Its Therapeutic Importance in Inflammation and Autoimmunity.Front Immunol. 2021 Apr 15;12:660342. doi: 10.3389/fimmu.2021.660342. eCollection 2021. Front Immunol. 2021. PMID: 33936095 Free PMC article. Review.
-
Insights Into Nicotinic Receptor Signaling in Nicotine Addiction: Implications for Prevention and Treatment.Curr Neuropharmacol. 2018;16(4):350-370. doi: 10.2174/1570159X15666170801103009. Curr Neuropharmacol. 2018. PMID: 28762314 Free PMC article. Review.
-
The influence of allosteric modulators and transmembrane mutations on desensitisation and activation of α7 nicotinic acetylcholine receptors.Neuropharmacology. 2015 Oct;97:75-85. doi: 10.1016/j.neuropharm.2015.05.006. Epub 2015 May 19. Neuropharmacology. 2015. PMID: 25998276 Free PMC article.
-
The 3,7-diazabicyclo[3.3.1]nonane scaffold for subtype selective nicotinic acetylcholine receptor (nAChR) ligands. Part 1: the influence of different hydrogen bond acceptor systems on alkyl and (hetero)aryl substituents.Bioorg Med Chem. 2013 Dec 1;21(23):7283-308. doi: 10.1016/j.bmc.2013.09.059. Epub 2013 Oct 5. Bioorg Med Chem. 2013. PMID: 24156938 Free PMC article.
References
-
- Changeux JP, Taly A. Nicotinic receptors, allosteric proteins and medicine. Trends Mol Med. 2008;14:93–102. - PubMed
-
- Tsetlin V, Kuzmin D, Kasheverov I. Assembly of nicotinic and other Cys-loop receptors. J Neurochem. 2011;116:734–741. - PubMed
-
- Karlin A. Emerging structure of the nicotinic acetylcholine receptors. Nat Rev Neurosci. 2002;3:102–114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
