Hepatic gene transfer in neonatal mice by adeno-associated virus serotype 8 vector
- PMID: 22098408
- PMCID: PMC3360497
- DOI: 10.1089/hum.2011.183
Hepatic gene transfer in neonatal mice by adeno-associated virus serotype 8 vector
Abstract
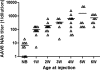
For genetic diseases that manifest at a young age with irreversible consequences, early treatment is critical and essential. Neonatal gene therapy has the advantages of achieving therapeutic effects before disease manifestation, a low vector requirement and high vector-to-cell ratio, and a relatively immature immune system. Therapeutic effects or long-term rescue of neonatal lethality have been demonstrated in several animal models. However, vigorous cell proliferation in the newborn stage is a significant challenge for nonintegrating vectors, such as adeno-associated viral (AAV) vector. Slightly delaying the injection age, and readministration at a later time, are two of the alternative strategies to solve this problem. In this study, we demonstrated robust and efficient hepatic gene transfer by self-complementary AAV8 vector in neonatal mice. However, transduction quickly decreased over a few weeks because of vector dilution caused by fast proliferation. Delaying the injection age improved sustained expression, although it also increased neutralizing antibody (NAb) responses to AAV capsid. This approach can be used to treat genetic diseases with slow progression. For genetic diseases with early onset and severe consequences, early treatment is essential. A second injection of vector of a different serotype at a later time may overcome preexisting NAb and achieve sustained therapeutic effects.
Figures




References
-
- Bell P. Moscioni A.D. McCarter R.J., et al. Analysis of tumors arising in male B6C3F1 mice with and without AAV vector delivery to liver. Mol. Ther. 2006;14:34–44. - PubMed
-
- Carbonaro D.A. Jin X. Petersen D., et al. In vivo transduction by intravenous injection of a lentiviral vector expressing human ADA into neonatal ADA gene knockout mice: A novel form of enzyme replacement therapy for ADA deficiency. Mol. Ther. 2006;13:1110–1120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

