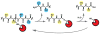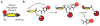Functional imaging of proteases: recent advances in the design and application of substrate-based and activity-based probes
- PMID: 22098719
- PMCID: PMC3237724
- DOI: 10.1016/j.cbpa.2011.10.012
Functional imaging of proteases: recent advances in the design and application of substrate-based and activity-based probes
Abstract
Proteases are enzymes that cleave peptide bonds in protein substrates. This process can be important for regulated turnover of a target protein but it can also produce protein fragments that then perform other functions. Because the last few decades of protease research have confirmed that proteolysis is an essential regulatory process in both normal physiology and in multiple disease-associated conditions, there has been an increasing interest in developing methods to image protease activity. Proteases are also considered to be one of the few 'druggable' classes of proteins and therefore a large number of small molecule based inhibitors of proteases have been reported. These compounds serve as a starting point for the design of probes that can be used to target active proteases for imaging applications. Currently, several classes of fluorescent probes have been developed to visualize protease activity in live cells and even whole organisms. The two primary classes of protease probes make use of either peptide/protein substrates or covalent inhibitors that produce a fluorescent signal when bound to an active protease target. This review outlines some of the most recent advances in the design of imaging probes for proteases. In particular, it highlights the strengths and weaknesses of both substrate-based and activity-based probes and their applications for imaging cysteine proteases that are important biomarkers for multiple human diseases.
Copyright © 2011 Elsevier Ltd. All rights reserved.
References
-
- Thornberry NA, Chapman KT, Nicholson DW. Determination of caspase specificities using a peptide combinatorial library. Methods Enzymol. 2000;322:100–10. - PubMed
-
- Los M, et al. Fluorogenic substrates as detectors of caspase activity during natural killer cell-induced apoptosis. Methods Mol Biol. 2000;121:155–62. - PubMed
-
- Gounaris E, et al. Live imaging of cysteine-cathepsin activity reveals dynamics of focal inflammation, angiogenesis, and polyp growth. PLoS One. 2008;3(8):e2916. ProSense680 was used to image cathepsin activity by intravital microscopy of intestinal lesions in mouse model of hereditary polyposis. Results indicate a link between protease activity, inflammation, and polyp growth. - PMC - PubMed
-
- Kozloff KM, et al. Non-invasive optical detection of cathepsin K-mediated fluorescence reveals osteoclast activity in vitro and in vivo. Bone. 2009;44(2):190–8. A polymer-based substrate probe was used to image Cathepsin K activity in osteoclast cultures and in mouse models of accelerated bone loss. Cathepsin K up-regulation could be imaged prior to bone loss detected by micro-CT, indicating its potential as a biomarker for early disease detection. - PMC - PubMed
-
- Nahrendorf M, et al. Dual channel optical tomographic imaging of leukocyte recruitment and protease activity in the healing myocardial infarct. Circ Res. 2007;100(8):1218–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources