Subdiffraction-limit study of Kaede diffusion and spatial distribution in live Escherichia coli
- PMID: 22098753
- PMCID: PMC3218334
- DOI: 10.1016/j.bpj.2011.10.013
Subdiffraction-limit study of Kaede diffusion and spatial distribution in live Escherichia coli
Abstract
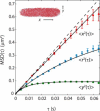
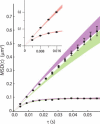
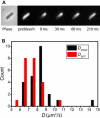
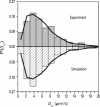
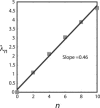
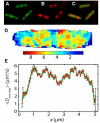
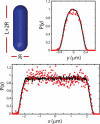
Photoactivation localization microscopy (PALM) is used to study the spatial distribution and diffusion of single copies of the protein Kaede in the cytoplasm of live Escherichia coli under moderate growth conditions (67 min doubling time). The spatial distribution of Kaede is uniform within the cytoplasm. The cytoplasmic radius of 380 ± 30 nm varies little from cell to cell. Single-particle tracking using 4 ms exposure times reveals negatively curved plots of mean-square displacement versus time. A detailed comparison with Monte Carlo simulations in a spherocylindrical volume shows that the curvature can be quantitatively understood in terms of free diffusion within a confining volume. The mean diffusion coefficient across cells is <D(Kaede)> = 7.3 ± 1.1 μm(2)·s(-1), consistent with a homotetrameric form of Kaede. The distribution of squared displacements along the long axis for individual Kaede molecules is consistent with homogeneous diffusion. However, for longer cells, a spatial map of one-step estimates of the diffusion coefficient along x suggests that diffusion is ∼20-40% faster within nucleoids than in the ribosome-rich region lying between nucleoid lobes at the cell mid-plane. Fluorescence recovery after photobleaching yielded <D(FRAP)> = 8.3 ± 1.6 μm(2)·s(-1), in agreement with the single-particle tracking results.
Copyright © 2011 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Betzig E., Patterson G.H., et al. Hess H.F. Imaging intracellular fluorescent proteins at nanometer resolution. Science. 2006;313:1642–1645. - PubMed
-
- Manley S., Gillette J.M., et al. Lippincott-Schwartz J. High-density mapping of single-molecule trajectories with photoactivated localization microscopy. Nat. Methods. 2008;5:155–157. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

