Directing migration of endothelial progenitor cells with applied DC electric fields
- PMID: 22099019
- PMCID: PMC3238468
- DOI: 10.1016/j.scr.2011.08.001
Directing migration of endothelial progenitor cells with applied DC electric fields
Abstract
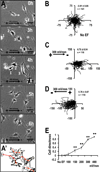
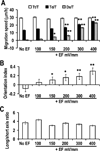
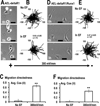
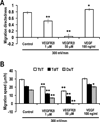
Naturally-occurring, endogenous electric fields (EFs) have been detected at skin wounds, damaged tissue sites and vasculature. Applied EFs guide migration of many types of cells, including endothelial cells to migrate directionally. Homing of endothelial progenitor cells (EPCs) to an injury site is important for repair of vasculature and also for angiogenesis. However, it has not been reported whether EPCs respond to applied EFs. Aiming to explore the possibility to use electric stimulation to regulate the progenitor cells and angiogenesis, we tested the effects of direct-current (DC) EFs on EPCs. We first used immunofluorescence to confirm the expression of endothelial progenitor markers in three lines of EPCs. We then cultured the progenitor cells in EFs. Using time-lapse video microscopy, we demonstrated that an applied DC EF directs migration of the EPCs toward the cathode. The progenitor cells also align and elongate in an EF. Inhibition of vascular endothelial growth factor (VEGF) receptor signaling completely abolished the EF-induced directional migration of the progenitor cells. We conclude that EFs are an effective signal that guides EPC migration through VEGF receptor signaling in vitro. Applied EFs may be used to control behaviors of EPCs in tissue engineering, in homing of EPCs to wounds and to an injury site in the vasculature.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures



Similar articles
-
Vascular endothelial growth factor-A signaling in bone marrow-derived endothelial progenitor cells exposed to hypoxic stress.Physiol Genomics. 2013 Nov 1;45(21):1021-34. doi: 10.1152/physiolgenomics.00070.2013. Epub 2013 Sep 10. Physiol Genomics. 2013. PMID: 24022223 Free PMC article.
-
A novel mechanism for endothelial progenitor cells homing: The SDF-1/CXCR4-Rac pathway may regulate endothelial progenitor cells homing through cellular polarization.Med Hypotheses. 2011 Feb;76(2):256-8. doi: 10.1016/j.mehy.2010.10.014. Epub 2010 Nov 4. Med Hypotheses. 2011. PMID: 21055881
-
Endothelial progenitor cells in angiogenesis.Sheng Li Xue Bao. 2005 Feb 25;57(1):1-6. Sheng Li Xue Bao. 2005. PMID: 15719128 Review.
-
Mobilization of endothelial progenitor cells in fracture healing and distraction osteogenesis.Bone. 2008 May;42(5):932-41. doi: 10.1016/j.bone.2008.01.007. Epub 2008 Jan 26. Bone. 2008. PMID: 18326482
-
The Role of Direct Current Electric Field-Guided Stem Cell Migration in Neural Regeneration.Stem Cell Rev Rep. 2016 Jun;12(3):365-75. doi: 10.1007/s12015-016-9654-8. Stem Cell Rev Rep. 2016. PMID: 27108005 Review.
Cited by
-
Voltage-gated ion channels mediate the electrotaxis of glioblastoma cells in a hybrid PMMA/PDMS microdevice.APL Bioeng. 2020 Jul 1;4(3):036102. doi: 10.1063/5.0004893. eCollection 2020 Sep. APL Bioeng. 2020. PMID: 32637857 Free PMC article.
-
Schwann cells promote endothelial cell migration.Cell Adh Migr. 2015;9(6):441-51. doi: 10.1080/19336918.2015.1103422. Cell Adh Migr. 2015. PMID: 26491999 Free PMC article.
-
The role of TGF-β in the electrotactic reaction of mouse 3T3 fibroblasts in vitro.Acta Biochim Pol. 2024 Jun 25;71:12993. doi: 10.3389/abp.2024.12993. eCollection 2024. Acta Biochim Pol. 2024. PMID: 38983797 Free PMC article.
-
Geometric constraints of endothelial cell migration on electrospun fibres.Sci Rep. 2018 Apr 23;8(1):6386. doi: 10.1038/s41598-018-24667-7. Sci Rep. 2018. PMID: 29686428 Free PMC article.
-
The effects of electrical stimulation on diabetic ulcers of foot and lower limb: A systematic review.Int Wound J. 2022 Nov;19(7):1911-1933. doi: 10.1111/iwj.13762. Epub 2022 Feb 2. Int Wound J. 2022. PMID: 35112496 Free PMC article.
References
-
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. - PubMed
-
- Akeson AL, Wetzel B, Thompson FY, Brooks SK, Paradis H, Gendron RL, Greenberg JM. Embryonic vasculogenesis by endothelial precursor cells derived from lung mesenchyme. Dev. Dyn. 2000;217:11–23. - PubMed
-
- Iwatsuki K, Tanaka K, Kaneko T, Kazama R, Okamoto S, Nakayama Y, Ito Y, Satake M, Takahashi S, Miyajima A, Watanabe T, Hara T. Runx1 promotes angiogenesis by downregulation of insulin-like growth factor-binding protein-3. Oncogene. 2005;24:1129–1137. - PubMed
-
- Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C, Ishida A, Fujita Y, Kothari S, Mohle R, Sauvage LR, Moore MA, Storb RF, Hammond WP. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92:362–367. - PubMed
-
- Zhao Z, Walczysko P, Zhao M. Intracellular Ca2+ stores are essential for injury induced Ca2+ signaling and re-endothelialization. J Cell Physiol. 2008;214:595–603. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

