Fine-tuning of Drp1/Fis1 availability by AKAP121/Siah2 regulates mitochondrial adaptation to hypoxia
- PMID: 22099302
- PMCID: PMC3360955
- DOI: 10.1016/j.molcel.2011.08.045
Fine-tuning of Drp1/Fis1 availability by AKAP121/Siah2 regulates mitochondrial adaptation to hypoxia
Abstract
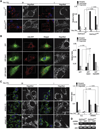
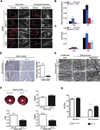
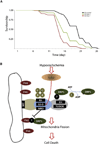
Defining the mechanisms underlying the control of mitochondrial fusion and fission is critical to understanding cellular adaptation to diverse physiological conditions. Here we demonstrate that hypoxia induces fission of mitochondrial membranes, dependent on availability of the mitochondrial scaffolding protein AKAP121. AKAP121 controls mitochondria dynamics through PKA-dependent inhibitory phosphorylation of Drp1 and PKA-independent inhibition of Drp1-Fis1 interaction. Reduced availability of AKAP121 by the ubiquitin ligase Siah2 relieves Drp1 inhibition by PKA and increases its interaction with Fis1, resulting in mitochondrial fission. High AKAP121 levels, seen in cells lacking Siah2, attenuate fission and reduce apoptosis of cardiomyocytes under simulated ischemia. Infarct size and degree of cell death were reduced in Siah2(-/-) mice subjected to myocardial infarction. Inhibition of Siah2 or Drp1 in hatching C. elegans reduces their life span. Through modulating Fis1/Drp1 complex availability, our studies identify Siah2 as a key regulator of hypoxia-induced mitochondrial fission and its physiological significance in ischemic injury and nematode life span.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures




References
-
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants and aging. Cell. 2005;120:483–495. - PubMed
-
- Carlucci A, Lignitto L, Feliciello A. Control of mytochondrial dynamics, oxidative metabolism by cAMP, AKAPs and proteasome. Trends Cell Biol. 2008b;18:604–613. - PubMed
-
- Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125:1241–1252. - PubMed
-
- Chang CR, Blackstone C. Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J. Biol. Chem. 2007;282:21583–21587. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

