Effect of exenatide on gastric emptying and graft survival in islet allograft recipients
- PMID: 22099764
- PMCID: PMC3262406
- DOI: 10.1016/j.transproceed.2011.10.022
Effect of exenatide on gastric emptying and graft survival in islet allograft recipients
Abstract
Objective: To evaluate the effect of exenatide on gastric emptying and long-term metabolic control.
Methods: Ten islet allograft recipients treated with exenatide up to 4 years. Data from a mixed meal test with (MMT+) versus without (MMT-) administration of exenatide before boost ingestion were analyzed at 6, 12, 24, 36, or 48 months after initiation of exenatide treatment. None of the subjects were symptomatic for gastroparesis before or during the study. The c-peptide, acetaminophen absorption and glucose responses to MMT were analyzed by Student t test and analysis of variance.
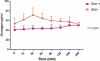
Results: Average exenatide dose was 12.75 ± 9.46 μg/dL. The MMT subjects included two groups those with acetaminophen peak ≤120 minutes ("good gastric emptying; n = 4") versus those with an acetaminophen peak ≥180 minutes ("delayed gastric emptying"). Among the MMT+, acetaminophen absorption was the same in both groups (P = .27). Up to 48 months exenatide delayed time to peak of glucose, c-peptide, and acetaminophen as well as suppressed the glucagon response to MMT mean peak: 70.89 ± 12.45 versus 43.24 ± 4.67. The mean values of c-peptide and glucose responses to MMT were not significantly different.
Conclusions: Long-term exenatide administration up to 4 years was safe in islet transplant recipients, even in the presence of delayed gastric emptying. The effects of exenatide were acute and reversible when the agent was withdrawn. The main difficulty with the use of exenatide in islet transplant subjects is their poor tolerability, although the physiological effects are clearly evident even at low doses. Approximately 63% of total subjects under exenatide treatment discontinued the drug due to nausea and vomiting. The use of new GLP1 analogs with longer half lives and fewer side effects may help to attain higher GLP1 levels, therefore improving islet function and survival.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
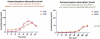

References
-
- Shapiro AM, Ricordi C, Hering BI, et al. International trial of the Edmonto protocol for islet transplantation. N Engl J Med. 2006;355:1318. - PubMed
-
- Faradji RN, Froud T, Pileggi A, et al. Islet alone and islet after kidney transplantation at the University of Miami: an update. Transplantation. 2006;82:340.
-
- Faradji RN, Monroy KP, Messinger S, et al. Simple measures to monitor β-cell mass and assess islet graft dysfunction. Am J Transplant. 2007;7:303. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

