The P4-ATPase TAT-5 inhibits the budding of extracellular vesicles in C. elegans embryos
- PMID: 22100064
- PMCID: PMC3237752
- DOI: 10.1016/j.cub.2011.10.040
The P4-ATPase TAT-5 inhibits the budding of extracellular vesicles in C. elegans embryos
Abstract
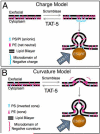
Background: Cells release extracellular vesicles (ECVs) that can influence differentiation, modulate the immune response, promote coagulation, and induce metastasis. Many ECVs form by budding outwards from the plasma membrane, but the molecules that regulate budding are unknown. In ECVs, the outer leaflet of the membrane bilayer contains aminophospholipids that are normally sequestered to the inner leaflet of the plasma membrane, suggesting a role for lipid asymmetry in ECV budding.
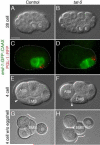
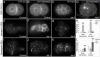
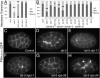
Results: We show that loss of the conserved P4-ATPase TAT-5 causes the large-scale shedding of ECVs and disrupts cell adhesion and morphogenesis in Caenorhabditis elegans embryos. TAT-5 localizes to the plasma membrane and its loss results in phosphatidylethanolamine exposure on cell surfaces. We show that RAB-11 and endosomal sorting complex required for transport (ESCRT) proteins, which regulate the topologically analogous process of viral budding, are enriched at the plasma membrane in tat-5 embryos, and are required for ECV production.
Conclusions: TAT-5 is the first protein identified to regulate ECV budding. TAT-5 provides a potential molecular link between loss of phosphatidylethanolamine asymmetry and the dynamic budding of vesicles from the plasma membrane, supporting the hypothesis that lipid asymmetry regulates budding. Our results also suggest that viral budding and ECV budding may share common molecular mechanisms.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures



Comment in
-
Extracellular vesicles: budding regulated by a phosphatidylethanolamine translocase.Curr Biol. 2011 Dec 20;21(24):R988-90. doi: 10.1016/j.cub.2011.11.009. Curr Biol. 2011. PMID: 22192830
References
-
- Tanaka Y, Okada Y, Hirokawa N. FGF-induced vesicular release of Sonic hedgehog and retinoic acid in leftward nodal flow is critical for left-right determination. Nature. 2005;435:172–177. - PubMed
-
- Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–593. - PubMed
-
- Basse F, Gaffet P, Rendu F, Bienvenue A. Translocation of spin-labeled phospholipids through plasma membrane during thrombin- and ionophore A23187-induced platelet activation. Biochemistry. 1993;32:2337–2344. - PubMed
-
- Morris RJ. Ionic control of the metastable inner leaflet of the plasma membrane: Fusions natural and artefactual. FEBS LETTERS. 2009;584:1665–1669. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

