Innate recognition of cell wall β-glucans drives invariant natural killer T cell responses against fungi
- PMID: 22100160
- PMCID: PMC5016029
- DOI: 10.1016/j.chom.2011.09.011
Innate recognition of cell wall β-glucans drives invariant natural killer T cell responses against fungi
Abstract
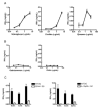
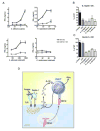
iNKT cells are innate T lymphocytes recognizing endogenous and foreign lipid antigens presented in the MHC-like molecule CD1d. The semi-invariant iNKT cell TCR can detect certain bacterial and parasitic lipids and drive iNKT cell responses. How iNKT cells respond to fungi, however, is unknown. We found that CD1d-deficient mice, which lack iNKT cells, poorly control infection with the fungal pathogen Aspergillus fumigatus. Furthermore, A. fumigatus rapidly activates iNKT cells in vivo and in vitro in the presence of APCs. Surprisingly, despite a requirement for CD1d recognition, the antifungal iNKT cell response does not require fungal lipids. Instead, Dectin-1- and MyD88-mediated responses to β-1,3 glucans, major fungal cell-wall polysaccharides, trigger IL-12 production by APCs that drives self-reactive iNKT cells to secrete IFN-γ. Innate recognition of β-1,3 glucans also drives iNKT cell responses against Candida, Histoplasma, and Alternaria, suggesting that this mechanism may broadly define the basis for antifungal iNKT cell responses.
Copyright © 2011 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no conflicting financial interests.
Figures
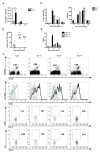
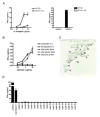
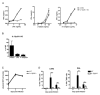
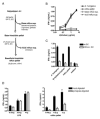

Comment in
-
iNKTs foil fungi.Cell Host Microbe. 2011 Nov 17;10(5):421-2. doi: 10.1016/j.chom.2011.11.003. Cell Host Microbe. 2011. PMID: 22100157 Free PMC article.
References
-
- Balloy V, Chignard M. The innate immune response to Aspergillus fumigatus. Microbes Infect. 2009;11:919–927. - PubMed
-
- Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. - PubMed
-
- Bernard M, Latge JP. Aspergillus fumigatus cell wall: composition and biosynthesis. Med Mycol. 2001;39(Suppl 1):9–17. - PubMed
-
- Brakhage AA, Bruns S, Thywissen A, Zipfel PF, Behnsen J. Interaction of phagocytes with filamentous fungi. Curr Opin Microbiol. 2010;13:409–415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

