Conditional deletion of Msx homeobox genes in the uterus inhibits blastocyst implantation by altering uterine receptivity
- PMID: 22100262
- PMCID: PMC3241866
- DOI: 10.1016/j.devcel.2011.09.010
Conditional deletion of Msx homeobox genes in the uterus inhibits blastocyst implantation by altering uterine receptivity
Abstract
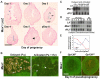
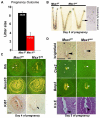
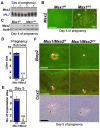
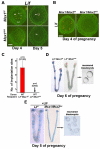
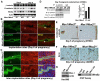
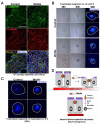
An effective bidirectional communication between an implantation-competent blastocyst and the receptive uterus is a prerequisite for mammalian reproduction. The blastocyst will implant only when this molecular cross-talk is established. Here we show that the muscle segment homeobox gene (Msh) family members Msx1 and Msx2, which are two highly conserved genes critical for epithelial-mesenchymal interactions during development, also play crucial roles in embryo implantation. Loss of Msx1/Msx2 expression correlates with altered uterine luminal epithelial cell polarity and affects E-cadherin/β-catenin complex formation through the control of Wnt5a expression. Application of Wnt5a in vitro compromised blastocyst invasion and trophoblast outgrowth on cultured uterine epithelial cells. The finding that Msx1/Msx2 genes are critical for conferring uterine receptivity and readiness to implantation could have clinical significance, because compromised uterine receptivity is a major cause of pregnancy failure in IVF programs.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

References
-
- Bach A, Lallemand Y, Nicola MA, Ramos C, Mathis L, Maufras M, Robert B. Msx1 is required for dorsal diencephalon patterning. Development. 2003;130:4025–4036. - PubMed
-
- Bei M, Maas R. FGFs and BMP4 induce both Msx1-independent and Msx1-dependent signaling pathways in early tooth development. Development. 1998;125:4325–4333. - PubMed
-
- Catron KM, Wang H, Hu G, Shen MM, Abate-Shen C. Comparison of MSX-1 and MSX-2 suggests a molecular basis for functional redundancy. Mech Dev. 1996;55:185–199. - PubMed
-
- Cha SW, Tadjuidje E, Tao Q, Wylie C, Heasman J. Wnt5a and Wnt11 interact in a maternal Dkk1-regulated fashion to activate both canonical and non-canonical signaling in Xenopus axis formation. Development. 2008;135:3719–3729. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

