Structural basis for activation of calcineurin by calmodulin
- PMID: 22100452
- PMCID: PMC3258384
- DOI: 10.1016/j.jmb.2011.11.008
Structural basis for activation of calcineurin by calmodulin
Abstract
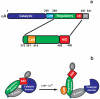
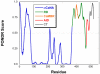
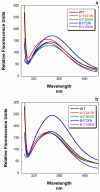
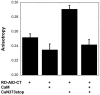
The highly conserved phosphatase calcineurin (CaN) plays vital roles in numerous processes including T-cell activation, development and function of the central nervous system, and cardiac growth. It is activated by the calcium sensor calmodulin (CaM). CaM binds to a regulatory domain (RD) within CaN, causing a conformational change that displaces an autoinhibitory domain (AID) from the active site, resulting in activation of the phosphatase. This is the same general mechanism by which CaM activates CaM-dependent protein kinases. Previously published data have hinted that the RD of CaN is intrinsically disordered. In this work, we demonstrate that the RD is unstructured and that it folds upon binding CaM, ousting the AID from the catalytic site. The RD is 95 residues long, with the AID attached to its C-terminal end and the 24-residue CaM binding region toward the N-terminal end. This is unlike the CaM-dependent protein kinases that have CaM binding sites and AIDs immediately adjacent in sequence. Our data demonstrate that not only does the CaM binding region folds but also an ∼25- to 30-residue region between it and the AID folds, resulting in over half of the RD adopting α-helical structure. This appears to be the first observation of CaM inducing folding of this scale outside of its binding site on a target protein.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures



References
-
- Wang JH, Desai R. A brain protein and its effect on the Ca2+-and protein modulator-activated cyclic nucleotide phosphodiesterase. Biochem Biophys Res Commun. 1976;72:926–932. - PubMed
-
- Watterson DM, Vanaman TC. Affinity chromatography purification of a cyclic nucleotide phosphodiesterase using immobilized modulator protein, a troponin C-like protein from brain. Biochem Biophys Res Commun. 1976;73:40–46. - PubMed
-
- Klee CB, Krinks MH. Purification of cyclic 3“,5-”nucleotide phosphodiesterase inhibitory protein by affinity chromatography on activator protein coupled to Sepharose. Biochemistry. 1978;17:120–126. - PubMed
-
- Rusnak F, Mertz P. Calcineurin: form and function. Physiol Rev. 2000;80:1483–1521. - PubMed
-
- Chakraborti S, Das S, Kar P, Ghosh B, Samanta K, Kolley S, Ghosh S, Roy S, Chakraborti T. Calcium signaling phenomena in heart diseases: a perspective. Mol Cell Biochem. 2007;298:1–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

