Rickettsia conorii infection stimulates the expression of ISG15 and ISG15 protease UBP43 in human microvascular endothelial cells
- PMID: 22100648
- PMCID: PMC3334299
- DOI: 10.1016/j.bbrc.2011.11.015
Rickettsia conorii infection stimulates the expression of ISG15 and ISG15 protease UBP43 in human microvascular endothelial cells
Abstract
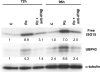
Rickettsia conorii, an obligate intracellular bacterium and the causative agent of Mediterranean spotted fever, preferentially infects microvascular endothelial cells of the mammalian hosts leading to onset of innate immune responses, characterized by the activation of intracellular signaling mechanisms, release of pro-inflammatory cytokines and chemokines, and killing of intracellular rickettsiae. Our recent studies have shown that interferon (IFN)-β, a cytokine traditionally considered to be involved in antiviral immunity, plays an important role in the autocrine/paracrine regulation of host defense mechanisms and control of R. conorii growth in the host endothelial cells. Here, we show that R. conorii infection induces the expression of ISG15 (an interferon-stimulated gene coding a protein of 17kD) and UBP43 (an ISG15-specific protease) at the levels of mRNA and protein and report the evidence of ISGylation of as yet unidentified target proteins in cultured human microvascular endothelium. Infection-induced expression of ISG15 and UBP43 requires intracellular replication of rickettsiae and production of IFN-β, because treatment with tetracycline and presence of an antibody capable of neutralizing IFN-β activity resulted in near complete attenuation of both responses. Inhibition of R. conorii-induced ISG15 by RNA interference results in significant increase in the extent of rickettsial replication, whereas UBP43 knockdown yields a reciprocal inhibitory effect. In tandem, these results demonstrate the stimulation of interferon-β-mediated innate immune mechanisms capable of perturbing the growth and replication of pathogenic rickettsiae and provide first evidence for ISG15-mediated post-translational modification of host cellular proteins during infection with an intracellular bacterium.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Suppressor of cytokine signalling protein SOCS1 and UBP43 regulate the expression of type I interferon-stimulated genes in human microvascular endothelial cells infected with Rickettsia conorii.J Med Microbiol. 2013 Jul;62(Pt 7):968-979. doi: 10.1099/jmm.0.054502-0. Epub 2013 Apr 4. J Med Microbiol. 2013. PMID: 23558133 Free PMC article.
-
Beta interferon-mediated activation of signal transducer and activator of transcription protein 1 interferes with Rickettsia conorii replication in human endothelial cells.Infect Immun. 2011 Sep;79(9):3733-43. doi: 10.1128/IAI.05008-11. Epub 2011 Jun 20. Infect Immun. 2011. PMID: 21690236 Free PMC article.
-
Endothelial Cell Proteomic Response to Rickettsia conorii Infection Reveals Activation of the Janus Kinase (JAK)-Signal Transducer and Activator of Transcription (STAT)-Inferferon Stimulated Gene (ISG)15 Pathway and Reprogramming Plasma Membrane Integrin/Cadherin Signaling.Mol Cell Proteomics. 2016 Jan;15(1):289-304. doi: 10.1074/mcp.M115.054361. Epub 2015 Nov 11. Mol Cell Proteomics. 2016. PMID: 26560068 Free PMC article.
-
Interferon-stimulated gene 15 and the protein ISGylation system.J Interferon Cytokine Res. 2011 Jan;31(1):119-30. doi: 10.1089/jir.2010.0110. Epub 2010 Dec 29. J Interferon Cytokine Res. 2011. PMID: 21190487 Free PMC article. Review.
-
Molecular Pathways of Interferon-Stimulated Gene 15: Implications in Cancer.Curr Protein Pept Sci. 2021;22(1):19-28. doi: 10.2174/1389203721999201208200747. Curr Protein Pept Sci. 2021. PMID: 33292152 Review.
Cited by
-
Enhancer Associated Long Non-coding RNA Transcription and Gene Regulation in Experimental Models of Rickettsial Infection.Front Immunol. 2019 Jan 9;9:3014. doi: 10.3389/fimmu.2018.03014. eCollection 2018. Front Immunol. 2019. PMID: 30687302 Free PMC article.
-
Global Transcriptomic Profiling of Pulmonary Gene Expression in an Experimental Murine Model of Rickettsia conorii Infection.Genes (Basel). 2019 Mar 8;10(3):204. doi: 10.3390/genes10030204. Genes (Basel). 2019. PMID: 30857242 Free PMC article.
-
Deubiquitinating enzymes as promising drug targets for infectious diseases.Curr Pharm Des. 2013;19(18):3234-47. doi: 10.2174/1381612811319180008. Curr Pharm Des. 2013. PMID: 23151130 Free PMC article. Review.
-
Cutaneous Immunoprofiles of Three Spotted Fever Group Rickettsia Cases.Infect Immun. 2020 Mar 23;88(4):e00686-19. doi: 10.1128/IAI.00686-19. Print 2020 Mar 23. Infect Immun. 2020. PMID: 31907196 Free PMC article.
-
Endothelial Cells as a Key Cell Type for Innate Immunity: A Focused Review on RIG-I Signaling Pathway.Front Immunol. 2022 Jul 5;13:951614. doi: 10.3389/fimmu.2022.951614. eCollection 2022. Front Immunol. 2022. PMID: 35865527 Free PMC article. Review.
References
-
- Leroy Q, Raoult D D. Mediterranean spotted fever. Infect. Dis. Clin. North Am. 2008;22:515–530. - PubMed
-
- Sousa R, França A, Dória Nòbrega S, Belo A, Amaro M, Abreu T, Poças J, Proença P, Vaz J, Torgal J, Bacellar F, Ismail N, Walker DH. Host- and microbe-related risk factors for and pathophysiology of fatal Rickettsia conorii infection in Portuguese patients. J. Infect. Dis. 2008;198:576–585. - PMC - PubMed
-
- Nicholson WL, Allen KE, McQuiston JH, Breitschwerdt EB, Little SE SE. The increasing recognition of rickettsial pathogens in dogs and people. Trends Parasitol. 2010;26:205–212. - PubMed
-
- Shah SS, McGowan JP. Rickettsial, ehrlichial and Bartonella infections of the myocardium and pericardium. Front. Biosci. 2003;8:e197–201. - PubMed
-
- Rombola F. Mediterranean spotted fever presenting as an acute pancreatitis. Acta Gastroenterol. Belg. 2011;74:91–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

