Epoxyeicosatrienoic acids and heme oxygenase-1 interaction attenuates diabetes and metabolic syndrome complications
- PMID: 22100745
- PMCID: PMC3261364
- DOI: 10.1016/j.prostaglandins.2011.10.002
Epoxyeicosatrienoic acids and heme oxygenase-1 interaction attenuates diabetes and metabolic syndrome complications
Abstract
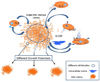
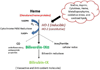
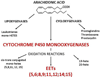
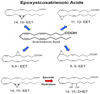
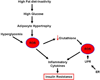
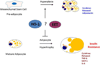
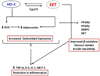
MSCs are considered to be the natural precursors to adipocyte development through the process of adipogenesis. A link has been established between decreased protective effects of EETs or HO-1 and their interaction in metabolic syndrome. Decreases in HO-1 or EET were associated with an increase in adipocyte stem cell differentiation and increased levels of inflammatory cytokines. EET agonist (AKR-I-27-28) inhibited MSC-derived adipocytes and decreased the levels of inflammatory cytokines. We further describe the role of CYP-epoxygenase expression, HO expression, and circulating cytokine levels in an obese mouse, ob/ob(-/-) mouse model. Ex vivo measurements of EET expression within MSCs derived from ob/ob(-/-) showed decreased levels of EETs that were increased by HO induction. This review demonstrates that suppression of HO and EET systems exist in MSCs prior to the development of adipocyte dysfunction. Further, adipocyte dysfunction can be ameliorated by induction of HO-1 and CYP-epoxygenase, i.e. EET.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures



References
-
- Peterson SJ, Frishman WH. Targeting heme oxygenase: therapeutic implications for diseases of the cardiovascular system. Cardiol Rev. 2009;17:99–111. - PubMed
-
- Li M, et al. Treatment of obese diabetic mice with a heme oxygenase inducer reduces visceral and subcutaneous adiposity, increases adiponectin levels, and improves insulin sensitivity and glucose tolerance. Diabetes. 2008;57:1526–1535. - PubMed
-
- Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev. 2008;60:79–127. - PubMed
-
- Abraham NG, Kappas A. Heme oxygenase and the cardiovascular-renal system. Free Radic Biol Med. 2005;39:1–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

