VDAC inhibition by tubulin and its physiological implications
- PMID: 22100746
- PMCID: PMC3302949
- DOI: 10.1016/j.bbamem.2011.11.004
VDAC inhibition by tubulin and its physiological implications
Abstract
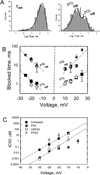
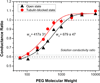
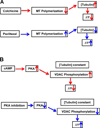
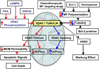
Regulation of mitochondrial outer membrane (MOM) permeability has dual importance: in normal metabolite and energy exchange between mitochondria and cytoplasm, and thus in control of respiration, and in apoptosis by release of apoptogenic factors into the cytosol. However, the mechanism of this regulation involving the voltage-dependent anion channel (VDAC), the major channel of MOM, remains controversial. For example, one of the long-standing puzzles was that in permeabilized cells, adenine nucleotide translocase is less accessible to cytosolic ADP than in isolated mitochondria. Still another puzzle was that, according to channel-reconstitution experiments, voltage regulation of VDAC is limited to potentials exceeding 30mV, which are believed to be much too high for MOM. We have solved these puzzles and uncovered multiple new functional links by identifying a missing player in the regulation of VDAC and, hence, MOM permeability - the cytoskeletal protein tubulin. We have shown that, depending on VDAC phosphorylation state and applied voltage, nanomolar to micromolar concentrations of dimeric tubulin induce functionally important reversible blockage of VDAC reconstituted into planar phospholipid membranes. The voltage sensitivity of the blockage equilibrium is truly remarkable. It is described by an effective "gating charge" of more than ten elementary charges, thus making the blockage reaction as responsive to the applied voltage as the most voltage-sensitive channels of electrophysiology are. Analysis of the tubulin-blocked state demonstrated that although this state is still able to conduct small ions, it is impermeable to ATP and other multi-charged anions because of the reduced aperture and inversed selectivity. The findings, obtained in a channel reconstitution assay, were supported by experiments with isolated mitochondria and human hepatoma cells. Taken together, these results suggest a previously unknown mechanism of regulation of mitochondrial energetics, governed by VDAC interaction with tubulin at the mitochondria-cytosol interface. Immediate physiological implications include new insights into serine/threonine kinase signaling pathways, Ca(2+) homeostasis, and cytoskeleton/microtubule activity in health and disease, especially in the case of the highly dynamic microtubule network which is characteristic of cancerogenesis and cell proliferation. In the present review, we speculate how these findings may help to identify new mechanisms of mitochondria-associated action of chemotherapeutic microtubule-targeting drugs, and also to understand why and how cancer cells preferentially use inefficient glycolysis rather than oxidative phosphorylation (Warburg effect). This article is part of a Special Issue entitled: VDAC structure, function, and regulation of mitochondrial metabolism.
Published by Elsevier B.V.
Figures



References
-
- Rostovtseva T, Colombini M. ATP flux is controlled by a voltage-gated channelfrom the mitochondrial outer membrane. J. Biol. Chem. 1996;271:28006–28008. - PubMed
-
- Colombini M. VDAC: The channel at the interface between mitochondria and thecytosol. Mol. Cell. Biochem. 2004;256:107–115. - PubMed
-
- Rostovtseva TK, Tan WZ, Colombini M. On the role of VDAC in apoptosis: Factand fiction. J. Bioenerg. Biomembr. 2005;37:129–142. - PubMed
-
- Shoshan-Barmatz V, Israelson A, Brdiczka D, Sheu SS. The voltage-dependent anion channel(VDAC): function in intracellular signaling, cell life and cell death. Curr. Pharm. Design. 2006;12:2249–2270. - PubMed
-
- Lemasters JJ, Holmuhamedov E. Voltage-dependent anion channel (VDAC) asmitochondrial governator - Thinking outside the box. Biochim. Biophys. Acta. 2006;1762:181–190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

