Bioactive silica-based nanoparticles stimulate bone-forming osteoblasts, suppress bone-resorbing osteoclasts, and enhance bone mineral density in vivo
- PMID: 22100753
- PMCID: PMC3291798
- DOI: 10.1016/j.nano.2011.11.003
Bioactive silica-based nanoparticles stimulate bone-forming osteoblasts, suppress bone-resorbing osteoclasts, and enhance bone mineral density in vivo
Abstract
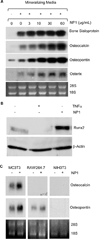
Bone is a dynamic tissue that undergoes renewal throughout life in a process whereby osteoclasts resorb worn bone and osteoblasts synthesize new bone. Imbalances in bone turnover lead to bone loss and development of osteoporosis and ultimately fracture, a debilitating condition with high morbidity and mortality. Silica is a ubiquitous biocontaminant that is considered to have high biocompatibility. The authors report that silica nanoparticles (NPs) mediate potent inhibitory effects on osteoclasts and stimulatory effects on osteoblasts in vitro. The mechanism of bioactivity is a consequence of an intrinsic capacity to antagonize activation of NF-κB, a signal transduction pathway required for osteoclastic bone resorption but inhibitory to osteoblastic bone formation. We further demonstrate that silica NPs promote a significant enhancement of bone mineral density (BMD) in mice in vivo, providing a proof of principle for the potential application of silica NPs as a pharmacological agent to enhance BMD and protect against bone fracture.
Published by Elsevier Inc.
Figures





References
-
- Riggs BL, Khosla S, Melton LJ., 3rd Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23:279–302. - PubMed
-
- Cho M, Cho WS, Choi M, Kim SJ, Han BS, Kim SH, et al. The impact of size on tissue distribution and elimination by single intravenous injection of silica nanoparticles. Toxicol Lett. 2009;189:177–183. - PubMed
-
- Bartneck M, Keul HA, Zwadlo-Klarwasser G, Groll J. Phagocytosis independent extracellular nanoparticle clearance by human immune cells. Nano Lett. 2010;10:59–63. - PubMed
-
- Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. - PubMed
-
- Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AR053607/AR/NIAMS NIH HHS/United States
- R21 AR053607/AR/NIAMS NIH HHS/United States
- CA136059/CA/NCI NIH HHS/United States
- AR056090/AR/NIAMS NIH HHS/United States
- R01 AR059364/AR/NIAMS NIH HHS/United States
- R03 CA136059/CA/NCI NIH HHS/United States
- R01 AR056090/AR/NIAMS NIH HHS/United States
- AR059364/AR/NIAMS NIH HHS/United States
- AG040013/AG/NIA NIH HHS/United States
- I01 BX000105/BX/BLRD VA/United States
- R01 CA136716/CA/NCI NIH HHS/United States
- R01 AG040013/AG/NIA NIH HHS/United States
- CA136716/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

