Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes
- PMID: 22100963
- PMCID: PMC3241321
- DOI: 10.2337/dc11-1438
Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes
Abstract
Objective: To determine whether short-time, real-time continuous glucose monitoring (RT-CGM) has long-term salutary glycemic effects in patients with type 2 diabetes who are not on prandial insulin.
Research design and methods: This was a randomized controlled trial of 100 adults with type 2 diabetes who were not on prandial insulin. This study compared the effects of 12 weeks of intermittent RT-CGM with self-monitoring of blood glucose (SMBG) on glycemic control over a 40-week follow-up period. Subjects received diabetes care from their regular provider without therapeutic intervention from the study team.
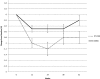
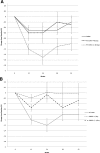
Results: There was a significant difference in A1C at the end of the 3-month active intervention that was sustained during the follow-up period. The mean, unadjusted A1C decreased by 1.0, 1.2, 0.8, and 0.8% in the RT-CGM group vs. 0.5, 0.5, 0.5, and 0.2% in the SMBG group at 12, 24, 38, and 52 weeks, respectively (P = 0.04). There was a significantly greater decline in A1C over the course of the study for the RT-CGM group than for the SMBG group, after adjusting for covariates (P < 0.0001). The subjects who used RT-CGM per protocol (≥48 days) improved the most (P < 0.0001). The improvement in the RT-CGM group occurred without a greater intensification of medication compared with those in the SMBG group.
Conclusions: Subjects with type 2 diabetes not on prandial insulin who used RT-CGM intermittently for 12 weeks significantly improved glycemic control at 12 weeks and sustained the improvement without RT-CGM during the 40-week follow-up period, compared with those who used only SMBG.
Figures
References
-
- National Diabetes Information Clearinghouse. National diabetes statistics [article online], 2011. Available from http://diabetes.niddk.nih.gov/dm/pubs/statistics/#diagnosed20 Accessed 29 May 2011
-
- Hoerger TJ, Segel JE, Gregg EW, Saaddine JB. Is glycemic control improving in U.S. adults? Diabetes Care 2008;31:81–86 - PubMed
-
- Nathan DM, Buse JB, Davidson MB, et al. ; American Diabetes Association European Association for Study of Diabetes Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32:193–203 - PMC - PubMed
-
- Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologist/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract 2009;15:540–559 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

