The triterpenoid CDDO-Me promotes hematopoietic progenitor expansion and myelopoiesis in mice
- PMID: 22100978
- PMCID: PMC3502000
- DOI: 10.1016/j.bbmt.2011.11.013
The triterpenoid CDDO-Me promotes hematopoietic progenitor expansion and myelopoiesis in mice
Abstract
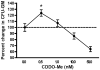
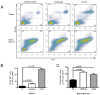
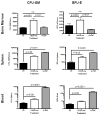
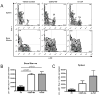
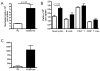
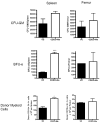
The synthetic triterpenoid CDDO-Me has been shown to directly inhibit the growth of myeloid leukemias and lends itself to a wide array of therapeutic indications, including inflammatory conditions, because of its inhibition of NF-κB. We have previously demonstrated protection from acute graft-versus-host disease after CDDO-Me administration in an allogeneic bone marrow transplantation model. In the current study, we observed that CDDO-Me promoted myelopoiesis in both naive and transplanted mice. This effect was dose dependent, as high doses of CDDO-Me inhibited myeloid growth in vitro. All lineages (granulocyte macrophage colony-forming unit, BFU-E) were promoted by CDDO-Me. We then compared the effects with granulocyte colony-stimulating factor, a known inducer of myeloid expansion and mobilization from the bone marrow. Whereas both drugs induced terminal myeloid expansion in the spleen, peripheral blood, and bone marrow, granulocyte colony-stimulating factor only induced granulocyte macrophage colony-forming unit precursors in the spleen, while CDDO-Me increased these precursors in the spleen and bone marrow. After sublethal total-body irradiation, mice pretreated with CDDO-Me further displayed an accelerated recovery of myeloid progenitors and total nucleated cells in the spleen. A similar expansion of myeloid and myeloid progenitors was noted with CDDO-Me treatment after syngeneic bone marrow transplantation. Combined, these data suggest that CDDO-Me may be of use posttransplantation to accelerate myeloid recovery in addition to the prevention of graft-versus-host disease.
Copyright © 2012 American Society for Blood and Marrow Transplantation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The remaining authors declare no competing financial interests.
Figures
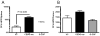
References
-
- Einsele H, Bertz H, Beyer J, et al. Infectious complications after allogeneic stem cell transplantation: epidemiology and interventional therapy strategies--guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO) Annals of hematology. 2003;82(Suppl 2):S175–185. - PMC - PubMed
-
- Weaver CH, Schwartzberg LS, Hainsworth J, et al. Treatment-related mortality in 1000 consecutive patients receiving high-dose chemotherapy and peripheral blood progenitor cell transplantation in community cancer centers. Bone marrow transplantation. 1997;19:671–678. - PubMed
-
- Trivedi M, Martinez S, Corringham S, Medley K, Ball ED. Optimal use of G-CSF administration after hematopoietic SCT. Bone marrow transplantation. 2009;43:895–908. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

