Neonatal Bisphenol A exposure alters sexually dimorphic gene expression in the postnatal rat hypothalamus
- PMID: 22101008
- PMCID: PMC3273679
- DOI: 10.1016/j.neuro.2011.11.002
Neonatal Bisphenol A exposure alters sexually dimorphic gene expression in the postnatal rat hypothalamus
Abstract
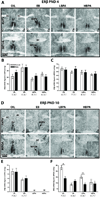
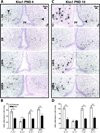
Developmental exposure to Bisphenol A (BPA), a component of polycarbonate and epoxy resins, has been purported to adversely impact reproductive function in female rodents. Because neonatal life is a critical window for the sexual dimorphic organization of the hypothalamic-pituitary-gonadal (HPG) axis, interference with this process could underlie compromised adult reproductive physiology. The goal of the present study was to determine if neonatal BPA exposure interferes with sex specific gene expression of estrogen receptor alpha (ERα), ER beta (ERβ) and kisspeptin (Kiss1) in the anterior and mediobasal hypothalamus. Long Evans (LE) neonatal rats were exposed to vehicle, 10μg estradiol benzoate (EB), 50mg/kg BPA or 50μg/kg BPA by subcutaneous injection daily from postnatal day 0 (PND 0) to PND 2. Gene expression was assessed by in situ hybridization on PNDs 4 and 10. Within the anterior hypothalamus ERα expression was augmented by BPA in PND 4 females, then fell to male-typical levels by PND 10. ERβ expression was not altered by BPA on PND 4, but significantly decreased or eliminated in both sexes by PND 10. Kiss1 expression was diminished by BPA in the anterior hypothalamus, especially in females. There were no significant impacts of BPA in the mediobasal hypothalamus. Collectively, BPA effects did not mirror those of EB. The results show that neonatal hypothalamic ER and Kiss1 expression is sensitive to BPA exposure. This disruption may alter sexually dimorphic hypothalamic organization and underlie adult reproductive deficiencies. Additionally, the discordant effects of EB and BPA indicate that BPA likely disrupts hypothalamic organization by a mechanism other than simply acting as an estrogen mimic.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures




References
-
- Aihara M, Hayashi S. Induction of persistent diestrus followed by persistent estrus is indicative of delayed maturation of tonic gonadotropin-releasing systems in rats. Biol Reprod. 1989;40:96–101. - PubMed
-
- Amateau SK, Alt JJ, Stamps CL, McCarthy MM. Brain estradiol content in newborn rats: sex differences, regional heterogeneity, and possible de novo synthesis by the female telencephalon. Endocrinology. 2004;145:2906–2917. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

