Nitrogen dioxide oxidizes mitochondrial cytochrome c
- PMID: 22101009
- PMCID: PMC3277883
- DOI: 10.1016/j.freeradbiomed.2011.09.024
Nitrogen dioxide oxidizes mitochondrial cytochrome c
Abstract
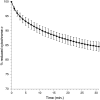
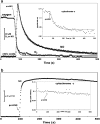
We previously reported that high micromolar concentrations of nitric oxide were able to oxidize mitochondrial cytochrome c at physiological pH, producing nitroxyl anion (Sharpe and Cooper, 1998 Biochem. J. 332, 9-19). However, the subsequent re-evaluation of the redox potential of the NO/NO(-) couple suggests that this reaction is thermodynamically unfavored. We now show that the oxidation is oxygen-concentration dependent and non stoichiometric. We conclude that the effect is due to an oxidant species produced during the aerobic decay of nitric oxide to nitrite and nitrate. The species is most probably nitrogen dioxide, NO(2)(•) a well-known biologically active oxidant. A simple kinetic model of NO autoxidation is able to explain the extent of cytochrome c oxidation assuming a rate constant of 3×10(6)M(-1)s(-1) for the reaction of NO(2)(•) with ferrocytochrome c. The importance of NO(2)(•) was confirmed by the addition of scavengers such as urate and ferrocyanide. These convert NO(2)(•) into products (urate radical and ferricyanide) that rapidly oxidize cytochrome c and hence greatly enhance the extent of oxidation observed. The present study does not support the previous hypothesis that NO and cytochrome c can generate appreciable amounts of nitroxyl ions (NO(-) or HNO) or of peroxynitrite.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures







References
-
- Stone J.R., Marletta M.A. Soluble guanylate cyclase from bovine lung: activation with nitric oxide and carbon monoxide and spectral characterization of the ferrous and ferric states. Biochemistry. 1994;33:5636–5640. - PubMed
-
- Brown G.C., Bolanos J.P., Heales S.J.R., Clark J.B. Nitric oxide produced by activated astrocytes rapidly and reversibly inhibits cellular respiration. Neurosci. Lett. 1995;193:201–204. - PubMed
-
- Victor V.M., Nunez C., D'Ocon P., Taylor C.T., Esplugues J.V., Moncada S. Regulation of oxygen distribution in tissues by endothelial nitric oxide. Circ. Res. 2009;104:1178–1183. - PubMed
-
- Keilin D., Hartree E.F. On some properties of catalase haematin. Proc. R. Soc. B. 1936;121:173–191.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

