Development of a new strategy for production of medium-chain-length polyhydroxyalkanoates by recombinant Escherichia coli via inexpensive non-fatty acid feedstocks
- PMID: 22101037
- PMCID: PMC3255744
- DOI: 10.1128/AEM.07020-11
Development of a new strategy for production of medium-chain-length polyhydroxyalkanoates by recombinant Escherichia coli via inexpensive non-fatty acid feedstocks
Abstract
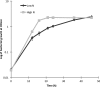
Pseudomonas putida KT2440 is capable of producing medium-chain-length polyhydroxyalkanoates (MCL-PHAs) when grown on unrelated carbon sources during nutrient limitation. Transcription levels of genes putatively involved in PHA biosynthesis were assessed by quantitative real-time PCR (qRT-PCR) in P. putida grown on glycerol as a sole carbon source. The results showed that two genes, phaG and the PP0763 gene, were highly upregulated among genes potentially involved in the biosynthesis of MCL-PHAs from unrelated carbon sources. Previous studies have described phaG as a 3-hydroxyacyl-acyl carrier protein (ACP)-coenzyme A (CoA) transferase, and based on homology, the PP0763 gene was predicted to encode a medium-chain-fatty-acid CoA ligase. High expression levels of these genes during PHA production in P. putida led to the hypothesis that these two genes are involved in PHA biosynthesis from non-fatty acid carbon sources, such as glucose and glycerol. The phaG(pp) and PP0763 genes from P. putida were cloned and coexpressed with the engineered Pseudomonas sp. 61-3 PHA synthase gene phaCl (STQK)(ps) in recombinant Escherichia coli. Up to 400 mg liter(-1) MCL-PHAs was successfully produced from glucose. This study has produced the largest amount of MCL-PHAs reported from non-fatty acid carbon sources in recombinant E. coli to date and opens up the possibility of using inexpensive feedstocks to produce MCL-PHA polymers.
Figures

References
-
- de Eugenio LI, et al. 2007. Biochemical evidence that phaZ gene encodes a specific intracellular medium chain length polyhydroxyalkanoate depolymerase in Pseudomonas putida KT2442: characterization of a paradigmatic enzyme. J. Biol. Chem. 282:4951–4962 - PubMed
-
- Fiedler S, Steinbuchel A, Rehm BH. 2002. The role of the fatty acid beta-oxidation multienzyme complex from Pseudomonas oleovorans in polyhydroxyalkanoate biosynthesis: molecular characterization of the fadBA operon from P. oleovorans and of the enoyl-CoA hydratase genes phaJ from P. oleovorans and Pseudomonas putida. Arch. Microbiol. 178:149–160 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

