Cultured representatives of two major phylogroups of human colonic Faecalibacterium prausnitzii can utilize pectin, uronic acids, and host-derived substrates for growth
- PMID: 22101049
- PMCID: PMC3255724
- DOI: 10.1128/AEM.06858-11
Cultured representatives of two major phylogroups of human colonic Faecalibacterium prausnitzii can utilize pectin, uronic acids, and host-derived substrates for growth
Abstract
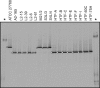
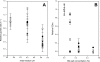
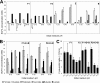
Faecalibacterium prausnitzii is one of the most abundant commensal bacteria in the healthy human large intestine, but information on genetic diversity and substrate utilization is limited. Here, we examine the phylogeny, phenotypic characteristics, and influence of gut environmental factors on growth of F. prausnitzii strains isolated from healthy subjects. Phylogenetic analysis based on the 16S rRNA sequences indicated that the cultured strains were representative of F. prausnitzii sequences detected by direct analysis of fecal DNA and separated the available isolates into two phylogroups. Most F. prausnitzii strains tested grew well under anaerobic conditions on apple pectin. Furthermore, F. prausnitzii strains competed successfully in coculture with two other abundant pectin-utilizing species, Bacteroides thetaiotaomicron and Eubacterium eligens, with apple pectin as substrate, suggesting that this species makes a contribution to pectin fermentation in the colon. Many F. prausnitzii isolates were able to utilize uronic acids for growth, an ability previously thought to be confined to Bacteroides spp. among human colonic anaerobes. Most strains grew on N-acetylglucosamine, demonstrating an ability to utilize host-derived substrates. All strains tested were bile sensitive, showing at least 80% growth inhibition in the presence of 0.5 μg/ml bile salts, while inhibition at mildly acidic pH was strain dependent. These attributes help to explain the abundance of F. prausnitzii in the colonic community but also suggest factors in the gut environment that may limit its distribution.
Figures


References
-
- Balamurugan R, Rajendiran E, George S, Samuel GV, Ramakrishna BS. 2008. Real-time polymerase chain reaction quantification of specific butyrate-producing bacteria, Desulfovibrio and Enterococcus faecalis in the feces of patients with colorectal cancer. J. Gastroenterol. Hepatol. 23:1298–1303 - PubMed
-
- Bryant MP. 1972. Commentary on the Hungate technique for cultivation of anaerobic bacteria. Am. J. Clin. Nutr. 25:1324–1328 - PubMed
-
- Cato EP, Salmon CW, Moore WEC. 1974. Fusobacterium prausnitzii (Hauduroy et al.) Moore and Holdeman: emended description and designation of neotype strain. Int. J. Syst. Bacteriol. 24:225–229
-
- Cowan S. 1974. Cowan and Steel's manual for the identification of medical bacteria, 2nd ed Cambridge University Press, London, United Kingdom
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

