Insights into phosphorylation-dependent mechanisms regulating USP1 protein stability during the cell cycle
- PMID: 22101265
- PMCID: PMC3272283
- DOI: 10.4161/cc.10.23.18501
Insights into phosphorylation-dependent mechanisms regulating USP1 protein stability during the cell cycle
Abstract
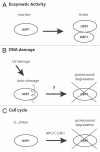
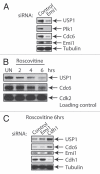
Tight regulation of the cell cycle and DNA repair machinery is essential for maintaining genome stability. The APC/CCdh1 ubiquitin ligase complex is a key regulator of protein stability during the G 1 phase of the cell cycle. APC/CCdh1 regulates and promotes the degradation of proteins involved in both cell cycle regulation and DNA repair. In a recent study, we identified a novel APC/CCdh1 substrate, the ubiquitin protease USP1. USP1 is a critical regulator of both the Fanconi anemia (FA) and translesion synthesis (TLS) DNA repair pathways. Here, we provide additional mechanistic insights into the regulation of USP1 during the cell cycle. Specifically, we demonstrate that USP1 is phosphorylated in mitosis by cyclin-dependent kinases (Cdks), and that this phosphorylation event may prevent premature degradation of USP1 during normal cell cycle progression. Finally, we provide a unifying hypothesis integrating the role of G 1-specific proteolysis of USP1 with the regulation of the transcriptional repressors, Inhibitor of DNA-binding (ID) proteins.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
