Drosophila endocytic neoplastic tumor suppressor genes regulate Sav/Wts/Hpo signaling and the c-Jun N-terminal kinase pathway
- PMID: 22101275
- PMCID: PMC3272291
- DOI: 10.4161/cc.10.23.18243
Drosophila endocytic neoplastic tumor suppressor genes regulate Sav/Wts/Hpo signaling and the c-Jun N-terminal kinase pathway
Abstract
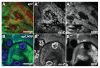
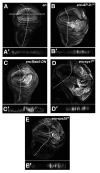
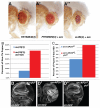
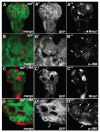
Genetic screens in the fruit fly Drosophila melanogaster have identified a class of neoplastic tumor suppressor genes (endocytic nTSGs), which encode proteins that localize to endosomes and facilitate the trafficking of membrane-bound receptors and adhesion molecules into the degradative lysosome. Loss of endocytic nTSGs transforms imaginal disc epithelia into highly proliferative, invasive tissues that fail to differentiate and display defects in cellular apicobasal polarity, adhesion and tissue architecture. As vertebrate homologs of some Drosophila nTSGs are linked to tumor formation, identifying molecular changes in signaling associated with nTSG loss could inform understanding of neoplastic transformation in vertebrates. Here we show that mutations in genes that act at multiple steps of the endolysosomal pathway lead to autonomous activation of the Sav/Wts/Hpo (SWH) transcriptional effector Yki (YAP/TAZ in vertebrates) and the Jun N-terminal kinase (JNK), which is known to promote Yki activity in cells with disrupted polarity. Yki and JNK activity are elevated by mutations at multiple steps in the endolysosomal pathway including mutations in the AP-2σ gene, which encodes a component of the AP-2 adaptor complex that recruits cargoes into clathrin-coated pits for subsequent internalization. Moreover, reduction of JNK activity can decrease elevated Yki-signaling caused by altered endocytosis. These studies reveal a broad requirement for components of the endocytic pathway in regulating SWH and JNK outputs, and place Drosophila endocytic nTSGs into a network that involving two major signaling pathways implicated in oncogenesis.
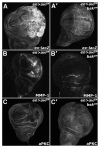
Figures

Similar articles
-
The cell adhesion molecule echinoid functions as a tumor suppressor and upstream regulator of the Hippo signaling pathway.Dev Cell. 2012 Feb 14;22(2):255-67. doi: 10.1016/j.devcel.2011.12.011. Epub 2012 Jan 25. Dev Cell. 2012. PMID: 22280890 Free PMC article.
-
Transcriptional output of the Salvador/warts/hippo pathway is controlled in distinct fashions in Drosophila melanogaster and mammalian cell lines.Cancer Res. 2009 Aug 1;69(15):6033-41. doi: 10.1158/0008-5472.CAN-08-4592. Epub 2009 Jul 7. Cancer Res. 2009. PMID: 19584286
-
Differential regulation of the Hippo pathway by adherens junctions and apical-basal cell polarity modules.Proc Natl Acad Sci U S A. 2015 Feb 10;112(6):1785-90. doi: 10.1073/pnas.1420850112. Epub 2015 Jan 26. Proc Natl Acad Sci U S A. 2015. PMID: 25624491 Free PMC article.
-
A role for Hipk in the Hippo pathway.Sci Signal. 2013 May 14;6(275):pe18. doi: 10.1126/scisignal.2004259. Sci Signal. 2013. PMID: 23674821 Review.
-
Lgl/aPKC and Crb regulate the Salvador/Warts/Hippo pathway.Fly (Austin). 2010 Oct-Dec;4(4):288-93. doi: 10.4161/fly.4.4.13116. Epub 2010 Oct 21. Fly (Austin). 2010. PMID: 20798605 Free PMC article. Review.
Cited by
-
De-regulation of JNK and JAK/STAT signaling in ESCRT-II mutant tissues cooperatively contributes to neoplastic tumorigenesis.PLoS One. 2013;8(2):e56021. doi: 10.1371/journal.pone.0056021. Epub 2013 Feb 13. PLoS One. 2013. PMID: 23418496 Free PMC article.
-
Roles of Membrane and Vesicular Traffic in Regulation of the Hippo Pathway.Front Cell Dev Biol. 2020 Jan 10;7:384. doi: 10.3389/fcell.2019.00384. eCollection 2019. Front Cell Dev Biol. 2020. PMID: 32010696 Free PMC article. Review.
-
The regulatory networks of the Hippo signaling pathway in cancer development.J Cancer. 2021 Aug 28;12(20):6216-6230. doi: 10.7150/jca.62402. eCollection 2021. J Cancer. 2021. PMID: 34539895 Free PMC article. Review.
-
JNK and Yorkie drive tumor progression by generating polyploid giant cells in Drosophila.Oncogene. 2018 Jun;37(23):3088-3097. doi: 10.1038/s41388-018-0201-8. Epub 2018 Mar 14. Oncogene. 2018. PMID: 29535423
-
Regulation of Notch signaling and endocytosis by the Lgl neoplastic tumor suppressor.Cell Cycle. 2015;14(10):1496-506. doi: 10.1080/15384101.2015.1026515. Cell Cycle. 2015. PMID: 25789785 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
