Cohesin acetyltransferase Esco2 is a cell viability factor and is required for cohesion in pericentric heterochromatin
- PMID: 22101327
- PMCID: PMC3252581
- DOI: 10.1038/emboj.2011.381
Cohesin acetyltransferase Esco2 is a cell viability factor and is required for cohesion in pericentric heterochromatin
Abstract
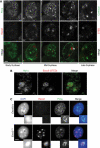
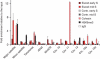
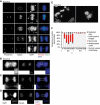
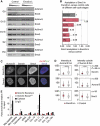
Sister chromatid cohesion, mediated by cohesin and regulated by Sororin, is essential for chromosome segregation. In mammalian cells, cohesion establishment and Sororin recruitment to chromatin-bound cohesin depends on the acetyltransferases Esco1 and Esco2. Mutations in Esco2 cause Roberts syndrome, a developmental disease in which mitotic chromosomes have a 'railroad' track morphology. Here, we show that Esco2 deficiency leads to termination of mouse development at pre- and post-implantation stages, indicating that Esco2 functions non-redundantly with Esco1. Esco2 is transiently expressed during S-phase when it localizes to pericentric heterochromatin (PCH). In interphase, Esco2 depletion leads to a reduction in cohesin acetylation and Sororin recruitment to chromatin. In early mitosis, Esco2 deficiency causes changes in the chromosomal localization of cohesin and its protector Sgo1. Our results suggest that Esco2 is needed for cohesin acetylation in PCH and that this modification is required for the proper distribution of cohesin on mitotic chromosomes and for centromeric cohesion.
Conflict of interest statement
The authors declare that they have no conflict of interest
Figures



References
-
- Ben-Shahar TR, Heeger S, Lehane C, East P, Flynn H, Skehel M, Uhlmann F (2008) Eco1-dependent cohesin acetylation during establishment of sister chromatid cohesion. Science 321: 563–566 - PubMed
-
- Gordillo M, Vega H, Trainer AH, Hou F, Sakai N, Luque R, Kayserili H, Basaran S, Skovby F, Hennekam RCM, Uzielli MLG, Schnur RE, Manouvrier S, Chang S, Blair E, Hurst JA, Forzano F, Meins M, Simola KOJ, Raas-Rothschild A et al. (2008) The molecular mechanism underlying Roberts syndrome involves loss of ESCO2 acetyltransferase activity. Hum Mol Genet 17: 2172–2180 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

