Phenotypic complexity of T regulatory subsets in patients with B-chronic lymphocytic leukemia
- PMID: 22101351
- PMCID: PMC3512204
- DOI: 10.1038/modpathol.2011.164
Phenotypic complexity of T regulatory subsets in patients with B-chronic lymphocytic leukemia
Abstract
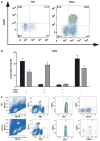
Increased numbers of T regulatory (T(reg)) cells are found in B-chronic lymphocytic leukemia, but the nature and function of these T(regs) remains unclear. Detailed characterization of the T(regs) in chronic lymphocytic leukemia has not been performed and the degree of heterogeneity of among these cells has not been studied to date. Using 15-color flow cytometry we show that T(reg) cells, defined using CD4, CD25, and forkhead box P3 (FOXP3), can be divided into multiple complex subsets based on markers used for naïve, memory, and effector delineation as well as markers of T(reg) activation. Furthermore FOXP3(+) cells can be identified among CD4(+)CD25(-) as well as CD8(+)CD4(-) populations in increased proportions in patients with chronic lymphocytic leukemia compared with healthy donors. Significantly different frequencies of naïve and effector T(regs) populations are found in healthy donor controls compared with donors with chronic lymphocytic leukemia. A population of CCR7(+)CD39(+) T(regs) was significantly associated with chronic lymphocytic leukemia. This population demonstrated slightly reduced suppressive activity compared with total T(regs) or T(regs) of healthy donors. These data suggest that FOXP3-expressing cells, particularly in patients with chronic lymphocytic leukemia are much more complex for T(reg) sub-populations and transitions than previously reported. These findings demonstrate the complexity of regulation of T-cell responses in chronic lymphocytic leukemia and illustrate the use of high-dimensional analysis of cellular phenotypes in facilitating understanding of the intricacies of cellular immune responses and their dysregulation in cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




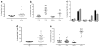
References
-
- Scrivener S, Goddard RV, Kaminski ER, Prentice AG. Abnormal T-cell function in B-cell chronic lymphocytic leukaemia. Leuk Lymphoma. 2003;44:383–389. - PubMed
-
- Reyes E, Prieto A, Carrion F, et al. Altered pattern of cytokine production by peripheral blood CD2+ cells from B chronic lymphocytic leukemia patients. Am J Hematol. 1998;57:93–100. - PubMed
-
- Dianzani U, Omede P, Marmont F, et al. Expansion of T cells expressing low CD4 or CD8 levels in B-cell chronic lymphocytic leukemia: correlation with disease status and neoplastic phenotype. Blood. 1994;83:2198–2205. - PubMed
-
- Mackus WJ, Frakking FN, Grummels A, et al. Expansion of CMV-specific CD8+CD45RA+CD27− T cells in B-cell chronic lymphocytic leukemia. Blood. 2003;102:1057–1063. - PubMed
-
- Beyer M, Kochanek M, Darabi K, et al. Reduced frequencies and suppressive function of CD4+CD25hi regulatory T cells in patients with chronic lymphocytic leukemia after therapy with fludarabine. Blood. 2005;106:2018–2025. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

