National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach
- PMID: 22101365
- PMCID: PMC3268003
- DOI: 10.1007/s00401-011-0910-3
National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach
Abstract
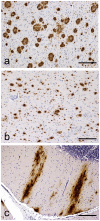
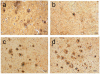
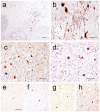
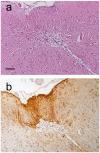
We present a practical guide for the implementation of recently revised National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease (AD). Major revisions from previous consensus criteria are: (1) recognition that AD neuropathologic changes may occur in the apparent absence of cognitive impairment, (2) an "ABC" score for AD neuropathologic change that incorporates histopathologic assessments of amyloid β deposits (A), staging of neurofibrillary tangles (B), and scoring of neuritic plaques (C), and (3) more detailed approaches for assessing commonly co-morbid conditions such as Lewy body disease, vascular brain injury, hippocampal sclerosis, and TAR DNA binding protein (TDP)-43 immunoreactive inclusions. Recommendations also are made for the minimum sampling of brain, preferred staining methods with acceptable alternatives, reporting of results, and clinico-pathologic correlations.
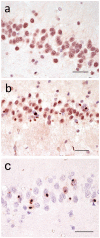
Figures

References
-
- Alafuzoff I, Parkkinen L, Al-Sarraj S, et al. Assessment of alpha-synuclein pathology: a study of the BrainNet Europe Consortium. J Neuropathol Exp Neurol. 2008;67:125–43. - PubMed
-
- Alafuzoff I, Pikkarainen M, Al-Sarraj S, et al. Interlaboratory comparison of assessments of Alzheimer disease-related lesions: a study of the BrainNet Europe Consortium. J Neuropathol Exp Neurol. 2006;65:740–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 AG013854/AG/NIA NIH HHS/United States
- P30 AG010124/AG/NIA NIH HHS/United States
- AG05131/AG/NIA NIH HHS/United States
- AG28383/AG/NIA NIH HHS/United States
- AG19610/AG/NIA NIH HHS/United States
- U19 AG024904/AG/NIA NIH HHS/United States
- AG24904/AG/NIA NIH HHS/United States
- AG12435/AG/NIA NIH HHS/United States
- AG13854/AG/NIA NIH HHS/United States
- P30 AG019610/AG/NIA NIH HHS/United States
- AG05134/AG/NIA NIH HHS/United States
- P30 AG028383/AG/NIA NIH HHS/United States
- R01 AG015819/AG/NIA NIH HHS/United States
- R01 AG042210/AG/NIA NIH HHS/United States
- RF1 AG015819/AG/NIA NIH HHS/United States
- AG17917/AG/NIA NIH HHS/United States
- AG10124/AG/NIA NIH HHS/United States
- P50 AG005131/AG/NIA NIH HHS/United States
- P30 AG062421/AG/NIA NIH HHS/United States
- U01 AG024904/AG/NIA NIH HHS/United States
- AG15866/AG/NIA NIH HHS/United States
- U19 AG010483/AG/NIA NIH HHS/United States
- AG03991/AG/NIA NIH HHS/United States
- U01 AG016976/AG/NIA NIH HHS/United States
- P01 AG003991/AG/NIA NIH HHS/United States
- P50 AG005681/AG/NIA NIH HHS/United States
- AG16570/AG/NIA NIH HHS/United States
- R01 AG017917/AG/NIA NIH HHS/United States
- AG18840/AG/NIA NIH HHS/United States
- R01 AG015866/AG/NIA NIH HHS/United States
- P50 AG005136/AG/NIA NIH HHS/United States
- P50 AG016570/AG/NIA NIH HHS/United States
- P50 AG005134/AG/NIA NIH HHS/United States
- AG15819/AG/NIA NIH HHS/United States
- P30 AG010161/AG/NIA NIH HHS/United States
- R01 AG018440/AG/NIA NIH HHS/United States
- P01 AG012435/AG/NIA NIH HHS/United States
- AG05681/AG/NIA NIH HHS/United States
- NS62684/NS/NINDS NIH HHS/United States
- P50 NS062684/NS/NINDS NIH HHS/United States
- AG05136/AG/NIA NIH HHS/United States
- AG10161/AG/NIA NIH HHS/United States
- P01 AG003949/AG/NIA NIH HHS/United States
- AG016976/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

