Central neural control of sympathetic nerve activity in heart failure following exercise training
- PMID: 22101524
- PMCID: PMC4120430
- DOI: 10.1152/ajpheart.00676.2011
Central neural control of sympathetic nerve activity in heart failure following exercise training
Abstract
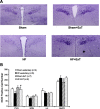
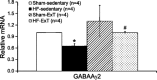
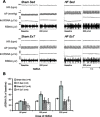
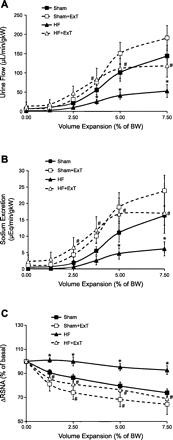
Typical characteristics of chronic congestive heart failure (HF) are increased sympathetic drive, altered autonomic reflexes, and altered body fluid regulation. These abnormalities lead to an increased risk of mortality, particularly in the late stage of chronic HF. Recent evidence suggests that central nervous system (CNS) mechanisms may be important in these abnormalities during HF. Exercise training (ExT) has emerged as a nonpharmacological therapeutic strategy substitute with significant benefit to patients with HF. Regular ExT improves functional capacity as well as quality of life and perhaps prognosis in chronic HF patients. The mechanism(s) by which ExT improves the clinical status of HF patients is not fully known. Recent studies have provided convincing evidence that ExT significantly alleviates the increased sympathetic drive, altered autonomic reflexes, and altered body fluid regulation in HF. This review describes and highlights the studies that examine various central pathways involved in autonomic outflow that are altered in HF and are improved following ExT. The increased sympathoexcitation is due to an imbalance between inhibitory and excitatory mechanisms within specific areas in the CNS such as the paraventricular nucleus (PVN) of the hypothalamus. Studies summarized here have revealed that ExT improves the altered inhibitory pathway utilizing nitric oxide and GABA mechanisms within the PVN in HF. ExT alleviates elevated sympathetic outflow in HF through normalization of excitatory glutamatergic and angiotensinergic mechanisms within the PVN. ExT also improves volume reflex function and thus fluid balance in HF. Preliminary observations also suggest that ExT induces structural neuroplasticity in the brain of rats with HF. We conclude that improvement of the enhanced CNS-mediated increase in sympathetic outflow, specifically to the kidneys related to fluid balance, contributes to the beneficial effects of ExT in HF.
Figures


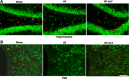
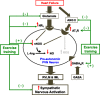
References
-
- Badoer E. Hypothalamic paraventricular nucleus and cardiovascular regulation. Clin Exp Pharmacol Physiol 28: 95–99, 2001 - PubMed
-
- Barbella Y, Cierco M, Israel A. Effect of Losartan, a nonpeptide angiotensin II receptor antagonist, on drinking behavior and renal actions of centrally administered renin. Proc Soc Exp Biol Med 202: 401–406, 1993 - PubMed
-
- Beatty JA, Kramer JM, Plowey ED, Waldrop TG. Physical exercise decreases neuronal activity in the posterior hypothalamic area of spontaneously hypertensive rats. J Appl Physiol 98: 572–578, 2005 - PubMed
-
- Chiodera P, Volpi R, Capretti L, Coiro V. Gamma-aminobutyric acid mediation of the inhibitory effect of nitric oxide on the arginine vasopressin and oxytocin responses to insulin-induced hypoglycemia. Regul Pept 67: 21–25, 1996 - PubMed
-
- Cooksey JD, Reilly P, Brown S, Bomze H, Cryer PE. Exercise training and plasma catecholamines in patients with ischemic heart disease. Am J Cardiol 42: 372–376, 1978 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

