Myofibroblasts cause heterogeneous Cx43 reduction and are unlikely to be coupled to myocytes in the healing canine infarct
- PMID: 22101526
- PMCID: PMC3353799
- DOI: 10.1152/ajpheart.00498.2011
Myofibroblasts cause heterogeneous Cx43 reduction and are unlikely to be coupled to myocytes in the healing canine infarct
Abstract
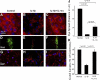
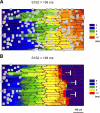
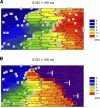
Following myocardial infarction (MI) inflammatory responses transform cardiac fibroblasts to myofibroblasts, which in vitro studies show form heterocellular gap junctions with cardiac myocytes via Connexin43 (Cx43). The ability to form heterocellular junctions in the intact heart and the impact of these junctions on propagation is unclear. We used a canine model of MI and characterized the distribution and quantity of myofibroblasts in surviving epicardial cells [epicardial border zone (EBZ)]. We found a significant increase in myofibroblasts within the EBZ and no gap junction plaques between myofibroblasts and myocytes. Because myofibroblasts produce IL-1β, which downregulates Cx43, we asked whether myofibroblast proliferation causes loss of Cx43 near myofibroblast clusters. In vitro studies showed that IL-1β caused loss of Cx43 and reduced coupling. Western blot showed a significant increase of IL-1β in the EBZ, and immunohistochemistry showed a loss of Cx43 in regions of myofibroblasts in the intact heart. Additionally, dye studies in intact heart showed no coupling between myocytes and myofibroblasts. To quantify the effect of myofibroblasts on propagation we used a two-dimensional subcellular computer model of the EBZ, which showed that heterogeneities in myofibroblast density lead to conduction abnormalities. In conclusion, an increase of myofibroblasts in the infarcted heart causes heterogeneous Cx43 levels, possibly as a result of the release of IL-1β and decreased cell-cell communication, which leads to conduction abnormalities following MI.
Figures








Similar articles
-
Cardiomyocyte-myofibroblast contact dynamism is modulated by connexin-43.FASEB J. 2019 Sep;33(9):10453-10468. doi: 10.1096/fj.201802740RR. Epub 2019 Jul 5. FASEB J. 2019. PMID: 31253057 Free PMC article.
-
Heterogeneous gap junction remodeling in reentrant circuits in the epicardial border zone of the healing canine infarct.Cardiovasc Res. 2006 Nov 1;72(2):241-9. doi: 10.1016/j.cardiores.2006.07.005. Epub 2006 Jul 12. Cardiovasc Res. 2006. PMID: 16914125
-
Electrical coupling between ventricular myocytes and myofibroblasts in the infarcted mouse heart.Cardiovasc Res. 2018 Mar 1;114(3):389-400. doi: 10.1093/cvr/cvx163. Cardiovasc Res. 2018. PMID: 29016731 Free PMC article.
-
New insights into myocardial arrhythmogenesis: distribution of gap-junctional coupling in normal, ischaemic and hypertrophied human hearts.Clin Sci (Lond). 1996 Jun;90(6):447-52. doi: 10.1042/cs0900447. Clin Sci (Lond). 1996. PMID: 8697713 Review.
-
Electrical consequences of cardiac myocyte: fibroblast coupling.Biochem Soc Trans. 2015 Jun;43(3):513-8. doi: 10.1042/BST20150035. Biochem Soc Trans. 2015. PMID: 26009200 Review.
Cited by
-
Emerging Arrhythmic Risk of Autoimmune and Inflammatory Cardiac Channelopathies.J Am Heart Assoc. 2018 Nov 20;7(22):e010595. doi: 10.1161/JAHA.118.010595. J Am Heart Assoc. 2018. PMID: 30571503 Free PMC article. Review. No abstract available.
-
Atrial fibrillation in cancer, anticancer therapies, and underlying mechanisms.J Mol Cell Cardiol. 2024 Sep;194:118-132. doi: 10.1016/j.yjmcc.2024.06.005. Epub 2024 Jun 17. J Mol Cell Cardiol. 2024. PMID: 38897563 Free PMC article. Review.
-
The Role of Pro-Inflammatory Cytokines in the Pathogenesis of Cardiovascular Disease.Int J Mol Sci. 2024 Jan 16;25(2):1082. doi: 10.3390/ijms25021082. Int J Mol Sci. 2024. PMID: 38256155 Free PMC article. Review.
-
Systemic Inflammation Rapidly Induces Reversible Atrial Electrical Remodeling: The Role of Interleukin-6-Mediated Changes in Connexin Expression.J Am Heart Assoc. 2019 Aug 20;8(16):e011006. doi: 10.1161/JAHA.118.011006. Epub 2019 Aug 19. J Am Heart Assoc. 2019. PMID: 31423933 Free PMC article.
-
Computational Approaches to Understanding the Role of Fibroblast-Myocyte Interactions in Cardiac Arrhythmogenesis.Biomed Res Int. 2015;2015:465714. doi: 10.1155/2015/465714. Epub 2015 Oct 25. Biomed Res Int. 2015. PMID: 26601107 Free PMC article. Review.
References
-
- Akar FG, Nass RD, Hahn S, Cingolani E, Shah M, Hesketh GG, DiSilvestre D, Tunin RS, Kass DA, Tomaselli GF. Dynamic changes in conduction velocity and gap junction properties during development of pacing-induced heart failure. Am J Physiol Heart Circ Physiol 293: H1223–H1230, 2007 - PubMed
-
- Angst BD, Khan LU, Severs NJ, Whitely K, Rothery S, Thompson RP, Magee AI, Gourdie RG. Dissociated spatial patterning of gap junctions and cell adhesion junctions during postnatal differentiation of ventricular myocardium. Circ Res 80: 88–94, 1997 - PubMed
-
- Cabo C, Boyden PA. Electrical remodeling of the epicardial border zone in the canine infarcted heart: a computational analysis. Am J Physiol Heart Circ Physiol 284: H372–H384, 2003 - PubMed
-
- Cabo C, Yao J, Boyden PA, Chen S, Hussain W, Duffy HS, Ciaccio EJ, Peters NS, Wit AL. Heterogeneous gap junction remodeling in reentrant circuits in the epicardial border zone of the healing canine infarct. Cardiovasc Res 72: 241–249, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

