Photoacclimation in Dunaliella tertiolecta reveals a unique NPQ pattern upon exposure to irradiance
- PMID: 22101577
- PMCID: PMC3224225
- DOI: 10.1007/s11120-011-9709-2
Photoacclimation in Dunaliella tertiolecta reveals a unique NPQ pattern upon exposure to irradiance
Abstract
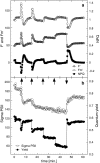
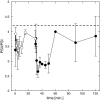
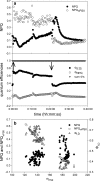
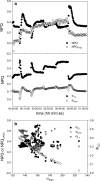
Highly time-resolved photoacclimation patterns of the chlorophyte microalga Dunaliella tertiolecta during exposure to an off-on-off (block) light pattern of saturating photon flux, and to a regime of consecutive increasing light intensities are presented. Non-photochemical quenching (NPQ) mechanisms unexpectedly responded with an initial decrease during dark-light transitions. NPQ values started to rise after light exposure of approximately 4 min. State-transitions, measured as a change of PSII:PSI fluorescence emission at 77 K, did not contribute to early NPQ oscillations. Addition of the uncoupler CCCP, however, caused a rapid increase in fluorescence and showed the significance of qE for NPQ. Partitioning of the quantum efficiencies showed that constitutive NPQ was (a) higher than qE-driven NPQ and (b) responded to light treatment within seconds, suggesting an active role of constitutive NPQ in variable energy dissipation, although it is thought to contribute statically to NPQ. The PSII connectivity parameter p correlated well with F', F(m)' and NPQ during the early phase of the dark-light transients in sub-saturating light, suggesting a plastic energy distribution pattern within energetically connected PSII centres. In consecutive increasing photon flux experiments, correlations were weaker during the second light increment. Changes in connectivity can present an early photoresponse that are reflected in fluorescence signals and NPQ and might be responsive to the short-term acclimation state, and/or to the actinic photon flux.
© The Author(s) 2011. This article is published with open access at Springerlink.com
Figures





References
-
- Adams WW, Demmig-Adams B. The xanthophyll cycle and sustained thermal energy dissipation activity in Vinca minor and Euonymus kiaufschovicus in winter. Plant Cell Environ. 1995;18(2):117–127.
-
- Adams WW, Demming-Adams B, Verhoeven AS, Barker D. Photoinhibition during winter stress-involvement of sustained xanthophyll cycle-dependent energy dissipation. Aust J Plant Physiol. 1995;22:261–276.
-
- Ahn TK, Avenson TJ, Peers G, Li Z, Dall’osto L, Bassi R, Niyogi KK, Fleming GR. Investigating energy partitioning during photosynthesis using an expanded quantum yield convention. Chem Phys. 2009;357(13):151–158.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

