Neural correlates of reliability-based cue weighting during multisensory integration
- PMID: 22101645
- PMCID: PMC3398428
- DOI: 10.1038/nn.2983
Neural correlates of reliability-based cue weighting during multisensory integration
Abstract
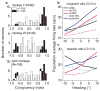
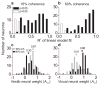
Integration of multiple sensory cues is essential for precise and accurate perception and behavioral performance, yet the reliability of sensory signals can vary across modalities and viewing conditions. Human observers typically employ the optimal strategy of weighting each cue in proportion to its reliability, but the neural basis of this computation remains poorly understood. We trained monkeys to perform a heading discrimination task from visual and vestibular cues, varying cue reliability randomly. The monkeys appropriately placed greater weight on the more reliable cue, and population decoding of neural responses in the dorsal medial superior temporal area closely predicted behavioral cue weighting, including modest deviations from optimality. We found that the mathematical combination of visual and vestibular inputs by single neurons is generally consistent with recent theories of optimal probabilistic computation in neural circuits. These results provide direct evidence for a neural mechanism mediating a simple and widespread form of statistical inference.
Figures





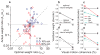
References
-
- Alais D, Burr D. The ventriloquist effect results from near-optimal bimodal integration. Curr Biol. 2004;14:257–262. - PubMed
-
- Ernst MO, Banks MS. Humans integrate visual and haptic information in a statistically optimal fashion. Nature. 2002;415:429–433. - PubMed
-
- Hillis JM, Watt SJ, Landy MS, Banks MS. Slant from texture and disparity cues: optimal cue combination. J Vis. 2004;4:967–992. - PubMed
-
- Jacobs RA. Optimal integration of texture and motion cues to depth. Vision Res. 1999;39:3621–3629. - PubMed
-
- Knill DC, Saunders JA. Do humans optimally integrate stereo and texture information for judgments of surface slant? Vision Res. 2003;43:2539–2558. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

