Crystallographic analysis of human hemoglobin elucidates the structural basis of the potent and dual antisickling activity of pyridyl derivatives of vanillin
- PMID: 22101818
- PMCID: PMC3211971
- DOI: 10.1107/S0907444911036353
Crystallographic analysis of human hemoglobin elucidates the structural basis of the potent and dual antisickling activity of pyridyl derivatives of vanillin
Erratum in
- Acta Crystallogr D Biol Crystallogr. 2011 Dec;67(Pt 12):1076
Abstract
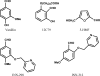
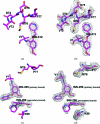
Vanillin has previously been studied clinically as an antisickling agent to treat sickle-cell disease. In vitro investigations with pyridyl derivatives of vanillin, including INN-312 and INN-298, showed as much as a 90-fold increase in antisickling activity compared with vanillin. The compounds preferentially bind to and modify sickle hemoglobin (Hb S) to increase the affinity of Hb for oxygen. INN-312 also led to a considerable increase in the solubility of deoxygenated Hb S under completely deoxygenated conditions. Crystallographic studies of normal human Hb with INN-312 and INN-298 showed that the compounds form Schiff-base adducts with the N-terminus of the α-subunits to constrain the liganded (or relaxed-state) Hb conformation relative to the unliganded (or tense-state) Hb conformation. Interestingly, while INN-298 binds and directs its meta-positioned pyridine-methoxy moiety (relative to the aldehyde moiety) further down the central water cavity of the protein, that of INN-312, which is ortho to the aldehyde, extends towards the surface of the protein. These studies suggest that these compounds may act to prevent sickling of SS cells by increasing the fraction of the soluble high-affinity Hb S and/or by stereospecific inhibition of deoxygenated Hb S polymerization.
© 2011 International Union of Crystallography. Printed in Singapore – all rights reserved.
Figures
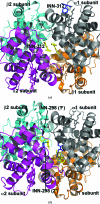

References
-
- Abdulmalik, O., Safo, M. K., Chen, Q., Yang, J., Brugnara, C., Ohene-Frempong, K., Abraham, D. J. & Asakura, T. (2005). Br. J. Haematol. 128, 552–561. - PubMed
-
- Abraham, D. J., Mehanna, A. S., Wireko, F. C., Whitney, J., Thomas, R. P. & Orringer, E. P. (1991). Blood, 77, 1334–1341. - PubMed
-
- Abraham, D. J., Safo, M. K., Boyiri, T., Danso-Danquah, R. E., Kister, J. & Poyart, C. (1995). Biochemistry, 34, 15006–15020. - PubMed
-
- Boyiri, T., Safo, M. K., Danso-Danquah, R. E., Kister, J., Poyart, C. & Abraham, D. J. (1995). Biochemistry, 34, 15021–15036. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

