Iron storage proteins are essential for the survival and pathogenesis of Mycobacterium tuberculosis in THP-1 macrophages and the guinea pig model of infection
- PMID: 22101841
- PMCID: PMC3264086
- DOI: 10.1128/JB.05553-11
Iron storage proteins are essential for the survival and pathogenesis of Mycobacterium tuberculosis in THP-1 macrophages and the guinea pig model of infection
Abstract
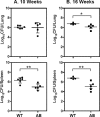
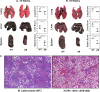
Iron is one of the crucial elements required for the growth of Mycobacterium tuberculosis. However, excess free iron becomes toxic for the cells because it catalyzes the production of reactive oxygen radicals, leading to oxidative damage. Hence, it is essential for the pathogen to have the ability to store intracellular iron in an iron-rich environment and utilize it under iron depletion. M. tuberculosis has two iron storage proteins, namely BfrA (Rv1876; a bacterioferritin) and BfrB (Rv3841; a ferritin-like protein). However, the demonstration of biological significance requires the disruption of relevant genes and the evaluation of the resulting mutant for its ability to survive in the host and cause disease. In this study, we have disrupted bfrA and bfrB of M. tuberculosis and demonstrated that these genes are crucial for the storage and supply of iron for the growth of bacteria and to withstand oxidative stress in vitro. In addition, the bfrA bfrB double mutant (H37Rv ΔbfrA ΔbfrB) exhibited a marked reduction in its ability to survive inside human macrophages. Guinea pigs infected with H37Rv ΔbfrA ΔbfrB exhibited a marked diminution in the dissemination of the bacilli to spleen compared to that of the parental strain. Moreover, guinea pigs infected with H37Rv ΔbfrA ΔbfrB exhibited significantly reduced pathological damage in spleen and lungs compared to that of animals infected with the parental strain. Our study clearly demonstrates the importance of these iron storage proteins in the survival and pathogenesis of M. tuberculosis in the host and establishes them as attractive targets for the development of new inhibitors against mycobacterial infections.
Figures

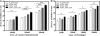


References
-
- Almirón MA, Ugalde RA. 2010. Iron homeostasis in Brucella abortus: the role of bacterioferritin. J. Microbiol. 48:668–673 - PubMed
-
- Andrews SC. 1998. Iron storage in bacteria. Adv. Microb. Physiol. 40:281–351 - PubMed
-
- Bardarov S, et al. 2002. Specialized transduction: an efficient method for generating marked and unmarked targeted gene disruptions in Mycobacterium tuberculosis, M. bovis BCG, and M. smegmatis. Microbiology 148:3007–3017 - PubMed
-
- Boelaert JR, Vandecasteele SJ, Appelberg R, Gordeuk VR. 2007. The effect of the host's iron status on tuberculosis. J. Infect. Dis. 195:1745–1753 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

