Role of Hedgehog signalling at the transition from double-positive to single-positive thymocyte
- PMID: 22101858
- PMCID: PMC3378705
- DOI: 10.1002/eji.201141758
Role of Hedgehog signalling at the transition from double-positive to single-positive thymocyte
Abstract
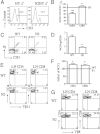
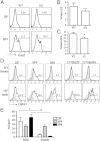
In the thymus, developing T cells receive signals that determine lineage choice, specificity, MHC restriction and tolerance to self-antigen. One way in which thymocytes receive instruction is by secretion of Sonic hedgehog (Shh) from thymic epithelial cells. We have previously shown that Hedgehog (Hh) signalling in the thymus decreases the CD4:CD8 single-positive (SP) thymocyte ratio. Here, we present data indicating that double-positive (DP) thymocytes are Hh-responsive and that thymocyte-intrinsic Hh signalling plays a role in modulating the production of CD4(+) (SP4), CD8(+) (SP8) and unconventional T-cell subsets. Repression of physiological Hh signalling in thymocytes altered the proportions of DP and SP4 cells. Thymocyte-intrinsic Hh-dependent transcription also attenuated both the production of mature SP4 and SP8 cells, and the establishment of peripheral T-cell compartments in TCR-transgenic mice. Additionally, stimulation or withdrawal of Hh signals in the WT foetal thymus impaired or enhanced upregulation of the CD4 lineage-specific transcription factor Gata3 respectively. These data together suggest that Hh signalling may play a role in influencing the later stages of thymocyte development.
Copyright © 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures





Similar articles
-
Sonic Hedgehog regulates thymic epithelial cell differentiation.J Autoimmun. 2016 Apr;68:86-97. doi: 10.1016/j.jaut.2015.12.004. Epub 2016 Jan 6. J Autoimmun. 2016. PMID: 26778835 Free PMC article.
-
Gli3 in fetal thymic epithelial cells promotes thymocyte positive selection and differentiation by repression of Shh.Development. 2018 Jan 31;145(3):dev146910. doi: 10.1242/dev.146910. Development. 2018. PMID: 29361554 Free PMC article.
-
The kinesin motor protein Kif7 is required for T-cell development and normal MHC expression on thymic epithelial cells (TEC) in the thymus.Oncotarget. 2017 Apr 11;8(15):24163-24176. doi: 10.18632/oncotarget.15241. Oncotarget. 2017. PMID: 28445929 Free PMC article.
-
Thymocyte migration and emigration.Immunol Lett. 2024 Jun;267:106861. doi: 10.1016/j.imlet.2024.106861. Epub 2024 Apr 30. Immunol Lett. 2024. PMID: 38697225 Review.
-
Transcriptional control of T-cell development.Int Immunol. 2011 Nov;23(11):661-8. doi: 10.1093/intimm/dxr078. Epub 2011 Sep 23. Int Immunol. 2011. PMID: 21948191 Review.
Cited by
-
The pioneer transcription factors Foxa1 and Foxa2 regulate alternative RNA splicing during thymocyte positive selection.Development. 2021 Aug 1;148(15):dev199754. doi: 10.1242/dev.199754. Epub 2021 Jul 29. Development. 2021. PMID: 34323272 Free PMC article.
-
Sonic Hedgehog regulates thymic epithelial cell differentiation.J Autoimmun. 2016 Apr;68:86-97. doi: 10.1016/j.jaut.2015.12.004. Epub 2016 Jan 6. J Autoimmun. 2016. PMID: 26778835 Free PMC article.
-
Gli3 in fetal thymic epithelial cells promotes thymocyte positive selection and differentiation by repression of Shh.Development. 2018 Jan 31;145(3):dev146910. doi: 10.1242/dev.146910. Development. 2018. PMID: 29361554 Free PMC article.
-
The transcriptional activator Gli2 modulates T-cell receptor signalling through attenuation of AP-1 and NFκB activity.J Cell Sci. 2015 Jun 1;128(11):2085-95. doi: 10.1242/jcs.165803. Epub 2015 Apr 23. J Cell Sci. 2015. PMID: 25908851 Free PMC article.
-
GATA binding protein 3 is correlated with leptin regulation of PPARγ1 in hepatic stellate cells.J Cell Mol Med. 2017 Mar;21(3):568-578. doi: 10.1111/jcmm.13002. Epub 2016 Oct 6. J Cell Mol Med. 2017. PMID: 27709831 Free PMC article.
References
-
- Wells FB, Gahm SJ, Hedrick SM, Bluestone JA, Dent A, Matis LA. Requirement for positive selection of gamma delta receptor-bearing T cells. Science. 1991;253:903–905. - PubMed
-
- Takahama Y. Journey through the thymus: stromal guides for T-cell development and selection. Nat. Rev. Immunol. 2006;6:127–135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

