Bmi1 reprograms CML B-lymphoid progenitors to become B-ALL-initiating cells
- PMID: 22101899
- PMCID: PMC3257014
- DOI: 10.1182/blood-2011-06-359232
Bmi1 reprograms CML B-lymphoid progenitors to become B-ALL-initiating cells
Abstract
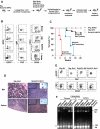
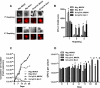
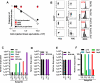
The characterization and targeting of Philadelphia chromosome positive (Ph(+)) acute lymphoblastic leukemia (ALL)-initiating cells remains unresolved. Expression of the polycomb protein Bmi1 is up-regulated in patients with advanced stages of chronic myelogenous leukemia (CML). We report that Bmi1 transforms and reprograms CML B-lymphoid progenitors into stem cell leukemia (Scl) promoter-driven, self-renewing, leukemia-initiating cells to result in B-lymphoid leukemia (B-ALL) in vivo. In vitro, highly proliferating and serially replatable myeloid and lymphoid colony-forming cultures could be established from BCR-ABL and Bmi1 coexpressing progenitors. However, unlike in vivo expanded CML B-lymphoid progenitors, hematopoietic stem cells, or multipotent progenitors, coexpressing BCR-ABL and Bmi1 did not initiate or propagate leukemia in a limiting dilution assay. Inducible genetic attenuation of BCR-ABL reversed Bmi1-driven B-ALL development, which was accompanied by induction of apoptosis of leukemic B-lymphoid progenitors and by long-term animal survival, suggesting that BCR-ABL is required to maintain B-ALL and that BCR-ABL and Bmi1 cooperate toward blast transformation in vivo. Our data indicate that BCR-ABL targeting itself is required to eradicate Ph(+)/Bmi1(+) B-ALL-initiating cells and confirm their addiction to BCR-ABL signaling.
Figures

References
-
- Notta F, Mullighan CG, Wang JC, et al. Evolution of human BCR-ABL1 lymphoblastic leukaemia-initiating cells. Nature. 2011;469(7330):362–367. - PubMed
-
- Smith KS, Chanda SK, Lingbeek M, et al. Bmi-1 regulation of INK4A-ARF is a downstream requirement for transformation of hematopoietic progenitors by E2a-Pbx1. Mol Cell. 2003;12(2):393–400. - PubMed
-
- Mullighan CG, Miller CB, Radtke I, et al. BCR-ABL1 lymphoblastic leukaemia is characterized by the deletion of Ikaros. Nature. 2008;453(7191):110–114. - PubMed
-
- Castor A, Nilsson L, Astrand-Grundstrom I, et al. Distinct patterns of hematopoietic stem cell involvement in acute lymphoblastic leukemia. Nat Med. 2005;11(6):630–637. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

