Mechanistic insights into the activation of oncogenic forms of EGF receptor
- PMID: 22101934
- PMCID: PMC3230693
- DOI: 10.1038/nsmb.2168
Mechanistic insights into the activation of oncogenic forms of EGF receptor
Abstract
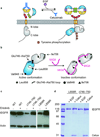
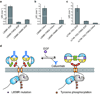
Epidermal growth factor receptor (EGFR) is a receptor tyrosine kinase that is commonly activated by mutation in non-small cell lung cancer. The mechanism of this oncogenic activation is not completely understood, but in contrast to that of the wild-type EGFR, it is proposed to be independent of kinase domain dimerization. Mechanistic studies on EGFR have mainly relied on cell-based assays or isolated kinase domain measurements. Here we show, using purified, near full-length human EGFR proteins (tEGFRs), that two oncogenic mutants are fully active independently of EGF and highly resistant to the therapeutic and endogenous inhibitors cetuximab, lapatinib and MIG6. Based on the pattern of inhibition and the effects of additional asymmetric kinase dimer interface mutations, we propose that these oncogenic EGFR mutants drive and strongly depend on the formation of the asymmetric kinase dimer for activation, which has implications for drug design and cancer treatment strategies.
Figures

Comment in
-
Finding the missing links in EGFR.Nat Struct Mol Biol. 2012 Jan 5;19(1):1-3. doi: 10.1038/nsmb.2221. Nat Struct Mol Biol. 2012. PMID: 22218287 No abstract available.
References
-
- Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. - PubMed
-
- Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. - PubMed
-
- Stamos J, Sliwkowski MX, Eigenbrot C. Structure of the epidermal growth factor receptor kinase domain alone and in complex with a 4-anilinoquinazoline inhibitor. J Biol Chem. 2002;277:46265–46272. - PubMed
-
- Lynch TJ, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

