Heterogeneity and dynamics of lateral line afferent innervation during development in zebrafish (Danio rerio)
- PMID: 22102005
- PMCID: PMC3377011
- DOI: 10.1002/cne.22798
Heterogeneity and dynamics of lateral line afferent innervation during development in zebrafish (Danio rerio)
Abstract
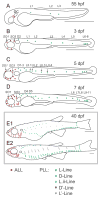
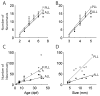
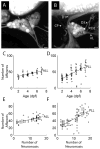
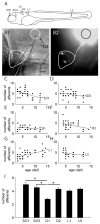
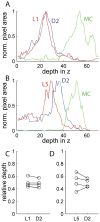
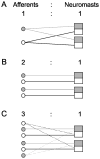
The lateral line system of larval zebrafish is emerging as a model to study a range of topics in neurobiology, from hair cell regeneration to sensory processing. However, despite numerous studies detailing the patterning and development of lateral line neuromasts, little is known about the organization of their connections to afferent neurons and targets in the hindbrain. We found that as fish grow and neuromasts proliferate over the body surface, the number of afferent neurons increases linearly. The number of afferents innervating certain neuromasts increases over time, while it decreases for other neuromasts. The ratio of afferent neurons to neuromasts differs between the anterior and posterior lateral line system, suggesting potential differences in sensitivity threshold or spatial resolution. A single afferent neuron routinely contacts a group of neuromasts, suggesting that different afferent neurons can convey information about receptive fields along the body. When afferent projections are traced into the hindbrain, where a distinct somatotopy has been previously described, we find that this general organization is absent at the Mauthner cell. We speculate that directional input from the lateral line is less important at an early age, whereas the speed of the escape response is paramount, and that directional responses arise later in development. By quantifying morphological connections in the lateral line system, this study provides a detailed foundation to understand how hydrodynamic information is processed and ultimately translated into appropriate motor behaviors.
Copyright © 2011 Wiley Periodicals, Inc.
Figures

Similar articles
-
Frequency response properties of primary afferent neurons in the posterior lateral line system of larval zebrafish.J Neurophysiol. 2015 Jan 15;113(2):657-68. doi: 10.1152/jn.00414.2014. Epub 2014 Oct 29. J Neurophysiol. 2015. PMID: 25355959 Free PMC article.
-
Physiology of afferent neurons in larval zebrafish provides a functional framework for lateral line somatotopy.J Neurophysiol. 2012 May;107(10):2615-23. doi: 10.1152/jn.01108.2011. Epub 2012 Feb 15. J Neurophysiol. 2012. PMID: 22338025 Free PMC article.
-
Neuronal birth order identifies a dimorphic sensorineural map.J Neurosci. 2012 Feb 29;32(9):2976-87. doi: 10.1523/JNEUROSCI.5157-11.2012. J Neurosci. 2012. PMID: 22378871 Free PMC article.
-
There and back again: development and regeneration of the zebrafish lateral line system.Wiley Interdiscip Rev Dev Biol. 2015 Jan-Feb;4(1):1-16. doi: 10.1002/wdev.160. Epub 2014 Oct 20. Wiley Interdiscip Rev Dev Biol. 2015. PMID: 25330982 Free PMC article. Review.
-
Developmental and architectural principles of the lateral-line neural map.Front Neural Circuits. 2013 Mar 26;7:47. doi: 10.3389/fncir.2013.00047. eCollection 2013. Front Neural Circuits. 2013. PMID: 23532704 Free PMC article. Review.
Cited by
-
Frequency response properties of primary afferent neurons in the posterior lateral line system of larval zebrafish.J Neurophysiol. 2015 Jan 15;113(2):657-68. doi: 10.1152/jn.00414.2014. Epub 2014 Oct 29. J Neurophysiol. 2015. PMID: 25355959 Free PMC article.
-
Afferent and motoneuron activity in response to single neuromast stimulation in the posterior lateral line of larval zebrafish.J Neurophysiol. 2014 Sep 15;112(6):1329-39. doi: 10.1152/jn.00274.2014. Epub 2014 Jun 25. J Neurophysiol. 2014. PMID: 24966296 Free PMC article.
-
An NIR emitting styryl dye with large Stokes shift to enable co-staining study on zebrafish neuromast hair cells.Bioorg Chem. 2019 Aug;89:103040. doi: 10.1016/j.bioorg.2019.103040. Epub 2019 Jun 3. Bioorg Chem. 2019. PMID: 31195328 Free PMC article.
-
Behavior, Electrophysiology, and Robotics Experiments to Study Lateral Line Sensing in Fishes.Integr Comp Biol. 2018 Nov 1;58(5):874-883. doi: 10.1093/icb/icy066. Integr Comp Biol. 2018. PMID: 29982706 Free PMC article. Review.
-
Physiology of afferent neurons in larval zebrafish provides a functional framework for lateral line somatotopy.J Neurophysiol. 2012 May;107(10):2615-23. doi: 10.1152/jn.01108.2011. Epub 2012 Feb 15. J Neurophysiol. 2012. PMID: 22338025 Free PMC article.
References
-
- Chiba A, Shepherd D, Murphey RK. Synaptic rearrangement during postembryonic development in the cricket. Science. 1988;240:901–905. - PubMed
-
- Corey DP, Hudspeth AJ. Ionic basis of the receptor potential in a vertebrate hair cell. Nature. 1979;281:675–677. - PubMed
-
- Cummings DM, Brunjes PC. The effects of variable periods of functional deprivation on olfactory bulb development in rats. Exp Neurol. 1997;148:360–366. - PubMed
-
- Faber DS, Korn H. Inputs from the posterior lateral line nerves upon the goldfish Mauthner cells. II. Evidence that the inhibitory components are mediated by interneurons of the recurrent collateral network. Brain Res. 1975;96:349–356. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

