Structure of New Delhi metallo-β-lactamase 1 (NDM-1)
- PMID: 22102018
- PMCID: PMC3212353
- DOI: 10.1107/S1744309111029654
Structure of New Delhi metallo-β-lactamase 1 (NDM-1)
Abstract
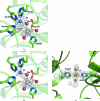
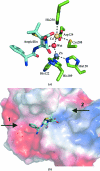
Antibiotic resistance in bacterial pathogens poses a serious threat to human health and the metallo-β-lactamase (MBL) enzymes are responsible for much of this resistance. The recently identified New Delhi MBL 1 (NDM-1) is a novel member of this family that is capable of hydrolysing a wide variety of clinically important antibiotics. Here, the crystal structure of NDM-1 from Klebsiella pneumoniae is reported and its structure and active site are discussed in the context of other recently deposited coordinates of NDM-1.
© 2011 International Union of Crystallography. All rights reserved.
Figures
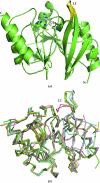
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

