Cleavage mediated by the P15 domain of bacterial RNase P RNA
- PMID: 22102593
- PMCID: PMC3299987
- DOI: 10.1093/nar/gkr1001
Cleavage mediated by the P15 domain of bacterial RNase P RNA
Abstract
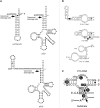
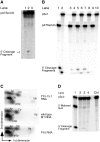
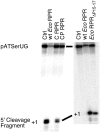
Independently folded domains in RNAs frequently adopt identical tertiary structures regardless of whether they are in isolation or are part of larger RNA molecules. This is exemplified by the P15 domain in the RNA subunit (RPR) of the universally conserved endoribonuclease P, which is involved in the processing of tRNA precursors. One of its domains, encompassing the P15 loop, binds to the 3'-end of tRNA precursors resulting in the formation of the RCCA-RNase P RNA interaction (interacting residues underlined) in the bacterial RPR-substrate complex. The function of this interaction was hypothesized to anchor the substrate, expose the cleavage site and result in re-coordination of Mg(2+) at the cleavage site. Here we show that small model-RNA molecules (~30 nt) carrying the P15-loop mediated cleavage at the canonical RNase P cleavage site with significantly reduced rates compared to cleavage with full-size RPR. These data provide further experimental evidence for our model that the P15 domain contributes to both substrate binding and catalysis. Our data raises intriguing evolutionary possibilities for 'RNA-mediated' cleavage of RNA.
Figures



Similar articles
-
Cleavage mediated by the catalytic domain of bacterial RNase P RNA.J Mol Biol. 2012 Sep 14;422(2):204-14. doi: 10.1016/j.jmb.2012.05.020. Epub 2012 May 22. J Mol Biol. 2012. PMID: 22626870
-
Functional coupling between a distal interaction and the cleavage site in bacterial RNase-P-RNA-mediated cleavage.J Mol Biol. 2011 Aug 12;411(2):384-96. doi: 10.1016/j.jmb.2011.05.049. Epub 2011 Jun 12. J Mol Biol. 2011. PMID: 21689663
-
Substrate recognition and cleavage-site selection by a single-subunit protein-only RNase P.Nucleic Acids Res. 2016 Mar 18;44(5):2323-36. doi: 10.1093/nar/gkw080. Epub 2016 Feb 20. Nucleic Acids Res. 2016. PMID: 26896801 Free PMC article.
-
Structure of ribonuclease P--a universal ribozyme.Curr Opin Struct Biol. 2006 Jun;16(3):327-35. doi: 10.1016/j.sbi.2006.04.002. Epub 2006 May 2. Curr Opin Struct Biol. 2006. PMID: 16650980 Review.
-
RNase P RNA mediated cleavage: substrate recognition and catalysis.Biochimie. 2007 Oct;89(10):1183-94. doi: 10.1016/j.biochi.2007.05.009. Epub 2007 Jun 2. Biochimie. 2007. PMID: 17624654 Review.
Cited by
-
The conformational space of RNase P RNA in solution.Nature. 2025 Jan;637(8048):1244-1251. doi: 10.1038/s41586-024-08336-6. Epub 2024 Dec 18. Nature. 2025. PMID: 39695229 Free PMC article.
-
Critical domain interactions for type A RNase P RNA catalysis with and without the specificity domain.PLoS One. 2018 Mar 6;13(3):e0192873. doi: 10.1371/journal.pone.0192873. eCollection 2018. PLoS One. 2018. PMID: 29509761 Free PMC article.
-
Inhibition of Bacterial RNase P RNA by Phenothiazine Derivatives.Biomolecules. 2016 Sep 8;6(3):38. doi: 10.3390/biom6030038. Biomolecules. 2016. PMID: 27618117 Free PMC article.
-
Piece by piece: Building a ribozyme.J Biol Chem. 2020 Feb 21;295(8):2313-2323. doi: 10.1074/jbc.REV119.009929. Epub 2020 Jan 17. J Biol Chem. 2020. PMID: 31953324 Free PMC article. Review.
-
Cleavage of Model Substrates by Arabidopsis thaliana PRORP1 Reveals New Insights into Its Substrate Requirements.PLoS One. 2016 Aug 5;11(8):e0160246. doi: 10.1371/journal.pone.0160246. eCollection 2016. PLoS One. 2016. PMID: 27494328 Free PMC article.
References
-
- Masquida B, Westhof E. A modular and hierarchial approach for all-atom RNA modeling. In: Gesteland RF, Cech TR, Atkins JF, editors. The RNA World. 3rd edn. Cold Spring Harbor, NY: Cold Spring Harbor Press; 2006. pp. 659–681.
-
- Hendrix DK, Brenner SE, Holbrook SR. RNA structural motifs: building blocks of a molecular biomolecule. Q. Rev. Biophys. 2005;38:221–243. - PubMed
-
- Michel F, Westhof E. Modelling of the three-dimensional architecture of group I catalytic introns based on comparative sequence analysis. J. Mol. Biol. 1990;216:585–610. - PubMed
-
- Murphy FL, Cech TR. An independently folding domain of RNA tertiary structure withion the Tetrahymna ribozyme. Biochemistry. 1993;32:5291–5300. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

