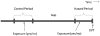Erythropoiesis-stimulating agents are not associated with increased risk of thrombosis in patients with myelodysplastic syndromes
- PMID: 22102702
- PMCID: PMC3248926
- DOI: 10.3324/haematol.2011.051755
Erythropoiesis-stimulating agents are not associated with increased risk of thrombosis in patients with myelodysplastic syndromes
Abstract
Background: There are limited reports of thrombosis among myelodysplastic syndrome patients exposed to erythropoiesis stimulating agents. It is not clear whether erythropoiesis stimulating agents are associated with an increased risk of thrombosis in myelodysplastic syndromes, as they are among patients with solid tumors.
Design and methods: The association between use of erythropoiesis stimulating agent and transient thrombosis risk in patients with myelodysplastic syndromes was assessed in a case-crossover study nested within a cohort of incident myelodysplastic syndrome patients. Using the US Surveillance, Epidemiology, and End Results Medicare-linked database, cases with an incident diagnosis of deep vein thrombosis were identified. Using conditional logistical regression, the odds of exposure to erythropoiesis stimulating agents in the 12 weeks prior to the incident deep vein thrombosis (hazard period) was compared to the exposure odds in a prior 12-week comparison period.
Results: Within the cohort of eligibles with myelodysplastic syndromes (n = 5,673) there were 212 incident cases of deep vein thrombosis events. Mean age was 76.2 (standard deviation = ± 8.6) years. Use of erythropoiesis stimulating agents was not associated with deep vein thrombosis in the crude nor the adjusted models (OR = 1.21, 95% CI: 0.60, 2.43). Central venous catheter placement (OR = 6.47, 95% CI: 2.37, 17.62) and red blood cell transfusion (OR = 4.60, 95% CI: 2.29, 9.23) were associated with deep vein thrombosis.
Conclusions: Despite the link between use of erythropoiesis stimulating agents and thrombosis among patients with solid tumors, this study provides evidence that their safety profile may be different among patients with myelodysplastic syndromes.
Figures
Similar articles
-
Efficacy and safety of luspatercept versus epoetin alfa in erythropoiesis-stimulating agent-naive, transfusion-dependent, lower-risk myelodysplastic syndromes (COMMANDS): interim analysis of a phase 3, open-label, randomised controlled trial.Lancet. 2023 Jul 29;402(10399):373-385. doi: 10.1016/S0140-6736(23)00874-7. Epub 2023 Jun 10. Lancet. 2023. PMID: 37311468 Clinical Trial.
-
Patient and physician characteristics associated with erythropoiesis-stimulating agent use in patients with myelodysplastic syndromes.Haematologica. 2012 Jan;97(1):128-32. doi: 10.3324/haematol.2011.049130. Haematologica. 2012. PMID: 22210329 Free PMC article.
-
Patterns of use and risks associated with erythropoiesis-stimulating agents among Medicare patients with cancer.J Natl Cancer Inst. 2009 Dec 2;101(23):1633-41. doi: 10.1093/jnci/djp387. Epub 2009 Nov 10. J Natl Cancer Inst. 2009. PMID: 19903808 Free PMC article.
-
Darbepoetin alfa for anemia with myelodysplastic syndrome.Expert Rev Hematol. 2015 Apr;8(2):139-46. doi: 10.1586/17474086.2015.1000854. Epub 2015 Jan 12. Expert Rev Hematol. 2015. PMID: 25579702 Review.
-
Erythropoiesis-stimulating agents versus RBC transfusion in MDS: comparison of long-term outcomes.Future Oncol. 2007 Aug;3(4):397-403. doi: 10.2217/14796694.3.4.397. Future Oncol. 2007. PMID: 17661714 Review.
Cited by
-
The economic burden of skeletal-related events among elderly men with metastatic prostate cancer.Pharmacoeconomics. 2014 Feb;32(2):173-91. doi: 10.1007/s40273-013-0121-y. Pharmacoeconomics. 2014. PMID: 24435407
-
Current therapy of myelodysplastic syndromes.Blood Rev. 2013 Sep;27(5):243-59. doi: 10.1016/j.blre.2013.07.003. Epub 2013 Jul 27. Blood Rev. 2013. PMID: 23954262 Free PMC article. Review.
-
Ischemic stroke as the initial presentation in acute myeloid leukemia vs. myelodysplastic syndrome: a case report and literature review with pathophysiological and clinical exploration.Neurol Sci. 2024 Jul;45(7):3297-3304. doi: 10.1007/s10072-024-07367-1. Epub 2024 Feb 14. Neurol Sci. 2024. PMID: 38351359 Review.
-
Thrombosis in Myeloid Malignancies: From CHIP to AML.Cardiovasc Hematol Disord Drug Targets. 2024;24(1):2-12. doi: 10.2174/011871529X307253240530060107. Cardiovasc Hematol Disord Drug Targets. 2024. PMID: 38879768 Review.
-
Resuscitating a dying marrow: the role of hematopoietic growth factors.Curr Hematol Malig Rep. 2014 Dec;9(4):412-20. doi: 10.1007/s11899-014-0236-z. Curr Hematol Malig Rep. 2014. PMID: 25311958 Review.
References
-
- Davidoff AJ, Weiss Smith S, Baer MR, Ke X, Bierenbaum JM, Hendrick F, et al. Access to Care, Race and Education Are Key Determinants of Erythropoietin Stimulating Agent (ESA) Use In Myelodysplastic Syndromes (MDS) Blood (ASH Annual Meeting Abstracts) 2010;116(21):3815.
-
- Berndt E, Kallich J, McDermott A, Xu X, Lee H, Glaspy J. Reductions in anaemia and fatigue are associated with improvements in productivity in cancer patients receiving chemotherapy. Pharmaco Economics. 2005;23(5):505–14. - PubMed
-
- Bohlius J, Langensiepen S, Schwarzer G, Seidenfeld J, Piper M, Bennett C, et al. Recombinant human erythropoietin and overall survival in cancer patients: results of a comprehensive meta-analysis. J Natl Cancer Inst. 2005;97(7):489–98. - PubMed
-
- Bohlius J, Schmidlin K, Brillant C, Schwarzer G, Trelle S, Seidenfeld J, et al. Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: a meta-analysis of randomised trials. Lancet. 2009;373(9674):1532–42. - PubMed
-
- Bohlius J, Wilson J, Seidenfeld J, Piper M, Schwarzer G, Sandercock J, et al. Recombinant human erythropoietins and cancer patients: updated meta-analysis of 57 studies including 9353 patients. J Natl Cancer Inst. 2006;98(10):708–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical