Deciphering the pathways of death of Histoplasma capsulatum-infected macrophages: implications for the immunopathogenesis of early infection
- PMID: 22102723
- PMCID: PMC3244509
- DOI: 10.4049/jimmunol.1102175
Deciphering the pathways of death of Histoplasma capsulatum-infected macrophages: implications for the immunopathogenesis of early infection
Abstract
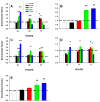
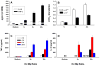
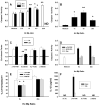
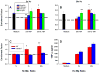
Apoptosis of leukocytes is known to strongly influence the immunopathogenesis of infection. In this study, we dissected the death pathways of murine macrophages (MΦs) infected with the intracellular pathogen Histoplasma capsulatum. Yeast cells caused apoptosis of MΦs at a wide range of multiplicity of infection, but smaller inocula resulted in delayed detection of apoptosis. Upon infection, caspases 3 and 1 were activated, and both contributed to cell death; however, only the former was involved in apoptosis. The principal driving force for apoptosis involved the extrinsic pathway via engagement of TNFR1 by TNF-α. Infected MΦs produced IL-10 that dampened apoptosis. The chronology of TNF-α and IL-10 release differed in vitro. The former was detected by 2 h postinfection, and the latter was not detected until 8 h postinfection. In vivo, the lungs of TNFR1(-/-) mice infected for 1 d contained fewer apoptotic MΦs than wild-type mice, whereas the lungs of IL-10(-/-) mice exhibited more. Blockade of apoptosis by a pan-caspase inhibitor or by simvastatin sharply reduced the release of TNF-α but enhanced IL-10. However, these treatments did not modify the fungal burden in vitro over 72 h. Thus, suppressing cell death modulated cytokine release but did not alter the fungal burden. These findings provide a framework for the early pathogenesis of histoplasmosis in which yeast cell invasion of lung MΦs engenders apoptosis, triggered in part in an autocrine TNF-α-dependent manner, followed by release of IL-10 that likely prevents apoptosis of newly infected neighboring phagocytes.
Figures

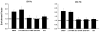
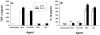

Similar articles
-
Apoptosis modulates protective immunity to the pathogenic fungus Histoplasma capsulatum.J Clin Invest. 2005 Oct;115(10):2875-85. doi: 10.1172/JCI25365. Epub 2005 Sep 8. J Clin Invest. 2005. PMID: 16151533 Free PMC article.
-
Regulation of infection with Histoplasma capsulatum by TNFR1 and -2.J Immunol. 2000 Sep 1;165(5):2657-64. doi: 10.4049/jimmunol.165.5.2657. J Immunol. 2000. PMID: 10946295
-
Protective and memory immunity to Histoplasma capsulatum in the absence of IL-10.J Immunol. 2003 Nov 15;171(10):5353-62. doi: 10.4049/jimmunol.171.10.5353. J Immunol. 2003. PMID: 14607938
-
Histoplasma capsulatum: master evader of innate immunity.Med Mycol J. 2014;55(4):E57-62. doi: 10.3314/mmj.55.E57. Med Mycol J. 2014. PMID: 25902570 Review. No abstract available.
-
Flying under the radar: Histoplasma capsulatum avoidance of innate immune recognition.Semin Cell Dev Biol. 2019 May;89:91-98. doi: 10.1016/j.semcdb.2018.03.009. Epub 2018 Mar 21. Semin Cell Dev Biol. 2019. PMID: 29551572 Free PMC article. Review.
Cited by
-
Comparative Proteomic Analysis of Histoplasma capsulatum Yeast and Mycelium Reveals Differential Metabolic Shifts and Cell Wall Remodeling Processes in the Different Morphotypes.Front Microbiol. 2021 Jun 11;12:640931. doi: 10.3389/fmicb.2021.640931. eCollection 2021. Front Microbiol. 2021. PMID: 34177824 Free PMC article.
-
Pro-Fibrotic Phenotype in a Patient with Segmental Stiff Skin Syndrome via TGF-β Signaling Overactivation.Int J Mol Sci. 2020 Jul 20;21(14):5141. doi: 10.3390/ijms21145141. Int J Mol Sci. 2020. PMID: 32698527 Free PMC article.
-
A Histoplasma capsulatum Lipid Metabolic Map Identifies Antifungal Targets.mBio. 2021 Dec 21;12(6):e0297221. doi: 10.1128/mBio.02972-21. Epub 2021 Nov 23. mBio. 2021. PMID: 34809453 Free PMC article.
-
An Intracellular Arrangement of Histoplasma capsulatum Yeast-Aggregates Generates Nuclear Damage to the Cultured Murine Alveolar Macrophages.Front Microbiol. 2016 Jan 11;6:1526. doi: 10.3389/fmicb.2015.01526. eCollection 2015. Front Microbiol. 2016. PMID: 26793172 Free PMC article.
-
Macrophage cell death and transcriptional response are actively triggered by the fungal virulence factor Cbp1 during H. capsulatum infection.Mol Microbiol. 2015 Dec;98(5):910-929. doi: 10.1111/mmi.13168. Epub 2015 Sep 29. Mol Microbiol. 2015. PMID: 26288377 Free PMC article.
References
-
- Opferman JT, Korsmeyer SJ. Apoptosis in the development and maintenance of the immune system. Nat Immunol. 2003;4:410–415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

