Eosinophils as a novel cell source of prostaglandin D2: autocrine role in allergic inflammation
- PMID: 22102725
- PMCID: PMC3237921
- DOI: 10.4049/jimmunol.1101806
Eosinophils as a novel cell source of prostaglandin D2: autocrine role in allergic inflammation
Abstract
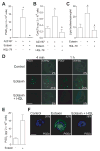
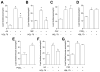
PGD(2) is a key mediator of allergic inflammatory diseases that is mainly synthesized by mast cells, which constitutively express high levels of the terminal enzyme involved in PGD(2) synthesis, the hematopoietic PGD synthase (H-PGDS). In this study, we investigated whether eosinophils are also able to synthesize, and therefore, supply biologically active PGD(2). PGD(2) synthesis was evaluated within human blood eosinophils, in vitro differentiated mouse eosinophils, and eosinophils infiltrating inflammatory site of mouse allergic reaction. Biological function of eosinophil-derived PGD(2) was studied by employing inhibitors of synthesis and activity. Constitutive expression of H-PGDS was found within nonstimulated human circulating eosinophils. Acute stimulation of human eosinophils with A23187 (0.1-5 μM) evoked PGD(2) synthesis, which was located at the nuclear envelope and was inhibited by pretreatment with HQL-79 (10 μM), a specific H-PGDS inhibitor. Prestimulation of human eosinophils with arachidonic acid (10 μM) or human eotaxin (6 nM) also enhanced HQL-79-sensitive PGD(2) synthesis, which, by acting on membrane-expressed specific receptors (D prostanoid receptors 1 and 2), displayed an autocrine/paracrine ability to trigger leukotriene C(4) synthesis and lipid body biogenesis, hallmark events of eosinophil activation. In vitro differentiated mouse eosinophils also synthesized paracrine/autocrine active PGD(2) in response to arachidonic acid stimulation. In vivo, at late time point of the allergic reaction, infiltrating eosinophils found at the inflammatory site appeared as an auxiliary PGD(2)-synthesizing cell population. Our findings reveal that eosinophils are indeed able to synthesize and secrete PGD(2), hence representing during allergic inflammation an extra cell source of PGD(2), which functions as an autocrine signal for eosinophil activation.
Figures



Similar articles
-
Leptin Elicits LTC4 Synthesis by Eosinophils Mediated by Sequential Two-Step Autocrine Activation of CCR3 and PGD2 Receptors.Front Immunol. 2018 Sep 20;9:2139. doi: 10.3389/fimmu.2018.02139. eCollection 2018. Front Immunol. 2018. PMID: 30298073 Free PMC article.
-
Co-operative signalling through DP(1) and DP(2) prostanoid receptors is required to enhance leukotriene C(4) synthesis induced by prostaglandin D(2) in eosinophils.Br J Pharmacol. 2011 Apr;162(8):1674-85. doi: 10.1111/j.1476-5381.2010.01086.x. Br J Pharmacol. 2011. PMID: 20973774 Free PMC article.
-
Cutting edge: prostaglandin D2 enhances leukotriene C4 synthesis by eosinophils during allergic inflammation: synergistic in vivo role of endogenous eotaxin.J Immunol. 2006 Feb 1;176(3):1326-30. doi: 10.4049/jimmunol.176.3.1326. J Immunol. 2006. PMID: 16424158
-
Anti- and proinflammatory effects of 15-deoxy-delta-prostaglandin J2(15d-PGJ2) on human eosinophil functions.Int Arch Allergy Immunol. 2007;143 Suppl 1:15-22. doi: 10.1159/000101399. Epub 2007 May 1. Int Arch Allergy Immunol. 2007. PMID: 17541271 Review.
-
Prostaglandin D₂ and T(H)2 inflammation in the pathogenesis of bronchial asthma.Korean J Intern Med. 2011 Mar;26(1):8-18. doi: 10.3904/kjim.2011.26.1.8. Epub 2011 Mar 2. Korean J Intern Med. 2011. PMID: 21437156 Free PMC article. Review.
Cited by
-
EicosaCell: An Imaging-Based Assay to Identify Spatiotemporal Eicosanoid Synthesis.Methods Mol Biol. 2017;1554:127-141. doi: 10.1007/978-1-4939-6759-9_6. Methods Mol Biol. 2017. PMID: 28185186 Free PMC article.
-
CRTH2 antagonists in asthma: current perspectives.Clin Pharmacol. 2017 Dec 15;9:165-173. doi: 10.2147/CPAA.S119295. eCollection 2017. Clin Pharmacol. 2017. PMID: 29276415 Free PMC article. Review.
-
Mucosal Type 2 Innate Lymphoid Cells Are a Key Component of the Allergic Response to Aeroallergens.Am J Respir Crit Care Med. 2017 Jun 15;195(12):1586-1596. doi: 10.1164/rccm.201609-1846OC. Am J Respir Crit Care Med. 2017. PMID: 28085492 Free PMC article.
-
Peripheral blood basophils are the main source for early interleukin-4 secretion upon in vitro stimulation with Culicoides allergen in allergic horses.PLoS One. 2021 May 26;16(5):e0252243. doi: 10.1371/journal.pone.0252243. eCollection 2021. PLoS One. 2021. PMID: 34038479 Free PMC article.
-
The role of prostaglandins in allergic lung inflammation and asthma.Expert Rev Respir Med. 2015 Feb;9(1):55-72. doi: 10.1586/17476348.2015.992783. Epub 2014 Dec 26. Expert Rev Respir Med. 2015. PMID: 25541289 Free PMC article. Review.
References
-
- Boyce JA. The role of mast cells in asthma. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2008;69:195–205. - PubMed
-
- Ogawa Y, Calhoun WJ. The role of leukotrienes in airway inflammation. J Allergy Clin Immunol. 2006;118:789–798. - PubMed
-
- Lee TH, Woszczek G, Farooque SP. Leukotriene E4: Perspective on the forgotten mediator. Journal of Allergy and Clinical Immunology. 2009;124:417–421. - PubMed
-
- Kanaoka Y, Urade Y. Hematopoietic prostaglandin D synthase. Prostaglandins Leukot Essent Fatty Acids. 2003;69:163–167. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

