Galactosaminogalactan, a new immunosuppressive polysaccharide of Aspergillus fumigatus
- PMID: 22102815
- PMCID: PMC3213105
- DOI: 10.1371/journal.ppat.1002372
Galactosaminogalactan, a new immunosuppressive polysaccharide of Aspergillus fumigatus
Abstract
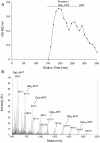
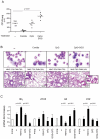
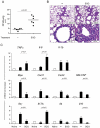
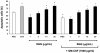
A new polysaccharide secreted by the human opportunistic fungal pathogen Aspergillus fumigatus has been characterized. Carbohydrate analysis using specific chemical degradations, mass spectrometry, ¹H and ¹³C nuclear magnetic resonance showed that this polysaccharide is a linear heterogeneous galactosaminogalactan composed of α1-4 linked galactose and α1-4 linked N-acetylgalactosamine residues where both monosacharides are randomly distributed and where the percentage of galactose per chain varied from 15 to 60%. This polysaccharide is antigenic and is recognized by a majority of the human population irrespectively of the occurrence of an Aspergillus infection. GalNAc oligosaccharides are an essential epitope of the galactosaminogalactan that explains the universal antibody reaction due to cross reactivity with other antigenic molecules containing GalNAc stretches such as the N-glycans of Campylobacter jejuni. The galactosaminogalactan has no protective effect during Aspergillus infections. Most importantly, the polysaccharide promotes fungal development in immunocompetent mice due to its immunosuppressive activity associated with disminished neutrophil infiltrates.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Morgan J, Wannemuehler KA, Marr KA, Hadley S, Kontoyiannis DP, et al. Incidence of invasive aspergillosis following hematopoietic stem cell and solid organ transplantation: interim results of a prospective multicenter surveillance program. Med Mycol. 2005;43(Suppl 1):S49–58. - PubMed
-
- Brakhage AA, Bruns S, Thywissen A, Zipfel PF, Behnsen J. Interaction of phagocytes with filamentous fungi. Curr Opin Microbiol. 2010;13:409–415. - PubMed
-
- Bozza S, Clavaud C, Giovannini G, Fontaine T, Beauvais A, et al. Immune sensing of Aspergillus fumigatus proteins, glycolipids, and polysaccharides and the impact on Th immunity and vaccination. J Immunol. 2009;183:2407–2414. - PubMed
-
- Mouyna I, Fontaine T. Cell wall of Aspergillus fumigatus: a dynamic structure. In: Latgé JP, Steinbach WJ, editors. Washington, D.C: American Society for Microbiology; 2009. pp. 169–183.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

