FOXO regulates organ-specific phenotypic plasticity in Drosophila
- PMID: 22102829
- PMCID: PMC3213149
- DOI: 10.1371/journal.pgen.1002373
FOXO regulates organ-specific phenotypic plasticity in Drosophila
Abstract
Phenotypic plasticity, the ability for a single genotype to generate different phenotypes in response to environmental conditions, is biologically ubiquitous, and yet almost nothing is known of the developmental mechanisms that regulate the extent of a plastic response. In particular, it is unclear why some traits or individuals are highly sensitive to an environmental variable while other traits or individuals are less so. Here we elucidate the developmental mechanisms that regulate the expression of a particularly important form of phenotypic plasticity: the effect of developmental nutrition on organ size. In all animals, developmental nutrition is signaled to growing organs via the insulin-signaling pathway. Drosophila organs differ in their size response to developmental nutrition and this reflects differences in organ-specific insulin-sensitivity. We show that this variation in insulin-sensitivity is regulated at the level of the forkhead transcription factor FOXO, a negative growth regulator that is activated when nutrition and insulin signaling are low. Individual organs appear to attenuate growth suppression in response to low nutrition through an organ-specific reduction in FOXO expression, thereby reducing their nutritional plasticity. We show that FOXO expression is necessary to maintain organ-specific differences in nutritional-plasticity and insulin-sensitivity, while organ-autonomous changes in FOXO expression are sufficient to autonomously alter an organ's nutritional-plasticity and insulin-sensitivity. These data identify a gene (FOXO) that modulates a plastic response through variation in its expression. FOXO is recognized as a key player in the response of size, immunity, and longevity to changes in developmental nutrition, stress, and oxygen levels. FOXO may therefore act as a more general regulator of plasticity. These data indicate that the extent of phenotypic plasticity may be modified by changes in the expression of genes involved in signaling environmental information to developmental processes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
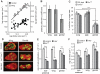
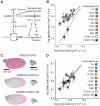
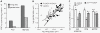

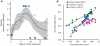
Comment in
-
Evo-devo: plastic flies.Nat Rev Genet. 2011 Nov 29;13(1):1. doi: 10.1038/nrg3131. Nat Rev Genet. 2011. PMID: 22124479 No abstract available.
References
-
- Samaras TT. Why the study of human size is important. In: Samaras TT, editor. Human Body Size and the Laws of Scaling. New York: Nova Science Publishers; 2007. pp. 1–15.
-
- Huber F. New Observations on Bees. London: Longman, Hurst, Rees, Orme and Brown; 1821.
-
- Edgar B. How flies get their size: genetics meets physiology. Nat Rev Genet. 2006;7:907–916. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

