Enhanced Actin Pedestal Formation by Enterohemorrhagic Escherichia coli O157:H7 Adapted to the Mammalian Host
- PMID: 22102844
- PMCID: PMC3219212
- DOI: 10.3389/fmicb.2011.00226
Enhanced Actin Pedestal Formation by Enterohemorrhagic Escherichia coli O157:H7 Adapted to the Mammalian Host
Abstract
Upon intestinal colonization, enterohemorrhagic Escherichia coli (EHEC) induces epithelial cells to generate actin "pedestals" beneath bound bacteria, lesions that promote colonization. To induce pedestals, EHEC utilizes a type III secretion system to translocate into the mammalian cell bacterial effectors such as translocated intimin receptor (Tir), which localizes in the mammalian cell membrane and functions as a receptor for the bacterial outer membrane protein intimin. Whereas EHEC triggers efficient pedestal formation during mammalian infection, EHEC cultured in vitro induces pedestals on cell monolayers with relatively low efficiency. To determine whether growth within the mammalian host enhances EHEC pedestal formation, we compared in vitro-cultivated bacteria with EHEC directly isolated from infected piglets. Mammalian adaptation by EHEC was associated with a dramatic increase in the efficiency of cell attachment and pedestal formation. The amounts of intimin and Tir were significantly higher in host-adapted than in in vitro-cultivated bacteria, but increasing intimin or Tir expression, or artificially increasing the level of bacterial attachment to mammalian cells, did not enhance pedestal formation by in vitro-cultivated EHEC. Instead, a functional assay suggested that host-adapted EHEC translocate Tir much more efficiently than does in vitro-cultivated bacteria. These data suggest that adaptation of EHEC to the mammalian intestine enhances bacterial cell attachment, expression of intimin and Tir, and translocation of effectors that promote actin signaling.
Keywords: EHEC; Tir; actin assembly; host adaptation; intimin; translocation.
Figures
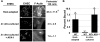



References
Grants and funding
LinkOut - more resources
Full Text Sources

