Early antiretroviral therapy during primary HIV-1 infection results in a transient reduction of the viral setpoint upon treatment interruption
- PMID: 22102898
- PMCID: PMC3216952
- DOI: 10.1371/journal.pone.0027463
Early antiretroviral therapy during primary HIV-1 infection results in a transient reduction of the viral setpoint upon treatment interruption
Abstract
Background: Long-term benefits of combination antiretroviral therapy (cART) initiation during primary HIV-1 infection are debated.
Methods: The evolution of plasma HIV-RNA (432 measurements) and cell-associated HIV-DNA (325 measurements) after cessation of cART (median exposure 18 months) was described for 33 participants from the Zurich Primary HIV Infection Study using linear regression and compared with 545 measurements from 79 untreated controls with clinically diagnosed primary HIV infection, respectively a known date for seroconversion.
Results: On average, early treated individuals were followed for 37 months (median) after cART cessation; controls had 34 months of pre-cART follow-up. HIV-RNA levels one year after cART interruption were -0.8 log₁₀ copies/mL [95% confidence interval -1.2;-0.4] lower in early treated patients compared with controls, but this difference was no longer statistically significant by year three of follow-up (-0.3 [-0.9; 0.3]). Mean HIV-DNA levels rebounded from 2 log₁₀ copies [1.8; 2.3] on cART to a stable plateau of 2.7 log₁₀ copies [2.5; 3.0] attained 1 year after therapy stop, which was not significantly different from cross-sectional measurements of 9 untreated members of the control group (2.8 log₁₀ copies [2.5; 3.1]).
Conclusions: The rebound dynamics of viral markers after therapy cessation suggest that early cART may indeed limit reservoir size of latently infected cells, but that much of the initial benefits are only transient. Owing to the non-randomized study design the observed treatment effects must be interpreted with caution.
Conflict of interest statement
Figures


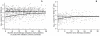
References
-
- Kinloch-de Loes S. Treatment of acute HIV-1 infection: is it coming of age? J Infect Dis. 2006;194:721–724. - PubMed
-
- Smith DE, Walker BD, Cooper DA, Rosenberg ES, Kaldor JM. Is antiretroviral treatment of primary HIV infection clinically justified on the basis of current evidence? AIDS. 2004;18:709–718. - PubMed
-
- Cellerai C, Little SJ, Loes SK. Treatment of acute HIV-1 infection: are we getting there? Curr Opin HIV AIDS. 2008;3:67–74. - PubMed
-
- Fidler S, Fox J, Touloumi G, Pantazis N, Porter K, et al. Slower CD4 cell decline following cessation of a 3 month course of HAART in primary HIV infection: findings from an observational cohort. AIDS. 2007;21:1283–1291. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

