Ebola virus glycoprotein needs an additional trigger, beyond proteolytic priming for membrane fusion
- PMID: 22102923
- PMCID: PMC3216919
- DOI: 10.1371/journal.pntd.0001395
Ebola virus glycoprotein needs an additional trigger, beyond proteolytic priming for membrane fusion
Abstract
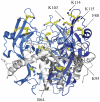
Background: Ebolavirus belongs to the family filoviridae and causes severe hemorrhagic fever in humans with 50-90% lethality. Detailed understanding of how the viruses attach to and enter new host cells is critical to development of medical interventions. The virus displays a trimeric glycoprotein (GP(1,2)) on its surface that is solely responsible for membrane attachment, virus internalization and fusion. GP(1,2) is expressed as a single peptide and is cleaved by furin in the host cells to yield two disulphide-linked fragments termed GP1 and GP2 that remain associated in a GP(1,2) trimeric, viral surface spike. After entry into host endosomes, GP(1,2) is enzymatically cleaved by endosomal cathepsins B and L, a necessary step in infection. However, the functional effects of the cleavage on the glycoprotein are unknown.
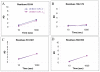
Principal findings: We demonstrate by antibody binding and Hydrogen-Deuterium Exchange Mass Spectrometry (DXMS) of glycoproteins from two different ebolaviruses that although enzymatic priming of GP(1,2) is required for fusion, the priming itself does not initiate the required conformational changes in the ectodomain of GP(1,2). Further, ELISA binding data of primed GP(1,2) to conformational antibody KZ52 suggests that the low pH inside the endosomes also does not trigger dissociation of GP1 from GP2 to effect membrane fusion.
Significance: The results reveal that the ebolavirus GP(1,2) ectodomain remains in the prefusion conformation upon enzymatic cleavage in low pH and removal of the glycan cap. The results also suggest that an additional endosomal trigger is necessary to induce the conformational changes in GP(1,2) and effect fusion. Identification of this trigger will provide further mechanistic insights into ebolavirus infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- World Health Organization. 2007. Ebola haemorrhagic fever in Uganda.
-
- Barrette RW, Metwally SA, Rowland JM, Xu L, Zaki SR, et al. Discovery of swine as a host for the Reston ebolavirus. Science. 2009;325:204–206. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS070899/NS/NINDS NIH HHS/United States
- AI081982/AI/NIAID NIH HHS/United States
- R01 AI081982/AI/NIAID NIH HHS/United States
- AI068730/AI/NIAID NIH HHS/United States
- R01 GM020501/GM/NIGMS NIH HHS/United States
- S10 RR029388/RR/NCRR NIH HHS/United States
- AI072106/AI/NIAID NIH HHS/United States
- GM020501/GM/NIGMS NIH HHS/United States
- R01 NS070899/NS/NINDS NIH HHS/United States
- GM093325/GM/NIGMS NIH HHS/United States
- R01 GM093325/GM/NIGMS NIH HHS/United States
- F32 GM020501/GM/NIGMS NIH HHS/United States
- GM066170/GM/NIGMS NIH HHS/United States
- AI2008031/AI/NIAID NIH HHS/United States
- RR029388/RR/NCRR NIH HHS/United States
- P01 AI068730/AI/NIAID NIH HHS/United States
- R01 AI072106/AI/NIAID NIH HHS/United States
- R01 GM066170/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

