Region-specific deletions of RIM1 reproduce a subset of global RIM1α(-/-) phenotypes
- PMID: 22103334
- PMCID: PMC3268893
- DOI: 10.1111/j.1601-183X.2011.00755.x
Region-specific deletions of RIM1 reproduce a subset of global RIM1α(-/-) phenotypes
Abstract
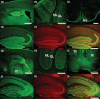
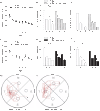
The presynaptic protein RIM1α mediates multiple forms of presynaptic plasticity at both excitatory and inhibitory synapses. Previous studies of mice lacking RIM1α (RIM1α(-/-) throughout the brain showed that deletion of RIM1α results in multiple behavioral abnormalities. In an effort to begin to delineate the brain regions in which RIM1 deletion mediates these abnormal behaviors, we used conditional (floxed) RIM1 knockout mice (fRIM1). By crossing these fRIM1 mice to previously characterized transgenic cre lines, we aimed to delete RIM1 selectively in the dentate gyrus (DG), using a specific preproopiomelanocortin promoter driving cre recombinase (POMC-cre) line , and in pyramidal neurons of the CA3 region of hippocampus, using the kainate receptor subunit 1 promoter driving cre recombinase (KA-cre). Neither of these cre driver lines was uniquely selective to the targeted regions. In spite of this, we were able to reproduce a subset of the global RIM1α(-/-) behavioral abnormalities, thereby narrowing the brain regions in which loss of RIM1 is sufficient to produce these behavioral differences. Most interestingly, hypersensitivity to the pyschotomimetic MK-801 was shown in mice lacking RIM1 selectively in the DG, arcuate nucleus of the hypothalamus and select cerebellar neurons, implicating novel brain regions and neuronal subtypes in this behavior.
© 2012 The Authors. Genes, Brain and Behavior © 2012 Blackwell Publishing Ltd and International Behavioural and Neural Genetics Society.
Figures







Similar articles
-
RIM1alpha and RIM1beta are synthesized from distinct promoters of the RIM1 gene to mediate differential but overlapping synaptic functions.J Neurosci. 2008 Dec 10;28(50):13435-47. doi: 10.1523/JNEUROSCI.3235-08.2008. J Neurosci. 2008. PMID: 19074017 Free PMC article.
-
RIM1alpha phosphorylation at serine-413 by protein kinase A is not required for presynaptic long-term plasticity or learning.Proc Natl Acad Sci U S A. 2008 Sep 23;105(38):14680-5. doi: 10.1073/pnas.0806679105. Epub 2008 Sep 17. Proc Natl Acad Sci U S A. 2008. PMID: 18799741 Free PMC article.
-
RIM1alpha and interacting proteins involved in presynaptic plasticity mediate prepulse inhibition and additional behaviors linked to schizophrenia.J Neurosci. 2010 Apr 14;30(15):5326-33. doi: 10.1523/JNEUROSCI.0328-10.2010. J Neurosci. 2010. PMID: 20392954 Free PMC article.
-
RIM function in short- and long-term synaptic plasticity.Biochem Soc Trans. 2005 Dec;33(Pt 6):1345-9. doi: 10.1042/BST0331345. Biochem Soc Trans. 2005. PMID: 16246115 Review.
-
Modeling the positive symptoms of schizophrenia in genetically modified mice: pharmacology and methodology aspects.Schizophr Bull. 2010 Mar;36(2):246-70. doi: 10.1093/schbul/sbp132. Epub 2009 Nov 9. Schizophr Bull. 2010. PMID: 19900963 Free PMC article. Review.
Cited by
-
Analysis of disease-associated objects at the Rat Genome Database.Database (Oxford). 2013 Jun 21;2013:bat046. doi: 10.1093/database/bat046. Print 2013. Database (Oxford). 2013. PMID: 23794737 Free PMC article.
-
Morris water maze: a versatile and pertinent tool for assessing spatial learning and memory.Exp Anim. 2022 Aug 5;71(3):264-280. doi: 10.1538/expanim.21-0120. Epub 2022 Mar 18. Exp Anim. 2022. PMID: 35314563 Free PMC article. Review.
-
The presynaptic active zone protein RIM1α controls epileptogenesis following status epilepticus.J Neurosci. 2012 Sep 5;32(36):12384-95. doi: 10.1523/JNEUROSCI.0223-12.2012. J Neurosci. 2012. PMID: 22956829 Free PMC article.
-
Cdk5/p35 functions as a crucial regulator of spatial learning and memory.Mol Brain. 2014 Nov 18;7:82. doi: 10.1186/s13041-014-0082-x. Mol Brain. 2014. PMID: 25404232 Free PMC article.
-
Beyond vertebrates: Drosophila melanogaster as a model to study negative symptoms of schizophrenia.Front Psychiatry. 2025 Jul 23;16:1622281. doi: 10.3389/fpsyt.2025.1622281. eCollection 2025. Front Psychiatry. 2025. PMID: 40778327 Free PMC article. Review.
References
-
- Akbarian S, Huang HS. Molecular and cellular mechanisms of altered GAD1/GAD67 expression in schizophrenia and related disorders. Brain Res Rev. 2006;52:293–304. - PubMed
-
- Balthasar N, Coppari R, Mcminn J, Liu SM, Lee CE, Tang V, Kenny CD, Mcgovern RA, Chua SC, Jr, Elmquist JK, Lowell BB. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. - PubMed
-
- Beasley CL, Reynolds GP. Parvalbumin-immunoreactive neurons are reduced in the prefrontal cortex of schizophrenics. Schizophr Res. 1997;24:349–355. - PubMed
-
- Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25:1–27. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

