Using infrared spectroscopy of cyanylated cysteine to map the membrane binding structure and orientation of the hybrid antimicrobial peptide CM15
- PMID: 22103476
- PMCID: PMC3246368
- DOI: 10.1021/bi200903p
Using infrared spectroscopy of cyanylated cysteine to map the membrane binding structure and orientation of the hybrid antimicrobial peptide CM15
Abstract
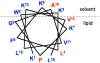
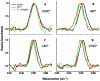
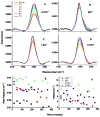
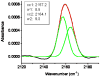
The synthetic antimicrobial peptide CM15, a hybrid of N-terminal sequences from cecropin and melittin peptides, has been shown to be extremely potent. Its mechanism of action has been thought to involve pore formation based on prior site-directed spin labeling studies. This study examines four single-site β-thiocyanatoalanine variants of CM15 in which the artificial amino acid side chain acts as a vibrational reporter of its local environment through the frequency and line shape of the unique CN stretching band in the infrared spectrum. Circular dichroism experiments indicate that the placements of the artificial side chain have only small perturbative effects on the membrane-bound secondary structure of the CM15 peptide. All variant peptides were placed in buffer solution, in contact with dodecylphosphatidylcholine micelles, and in contact with vesicles formed from Escherichia coli polar lipid extract. At each site, the CN stretching band reports a different behavior. Time-dependent attenuated total reflectance infrared spectra were also collected for each variant as it was allowed to remodel the E. coli lipid vesicles. The results of these experiments agree with the previously proposed formation of toroidal pores, in which each peptide finds itself in an increasingly homogeneous and curved local environment without apparent peptide-peptide interactions. This work also demonstrates the excellent sensitivity of the SCN stretching vibration to small changes in the peptide-lipid interfacial structure.
Figures


References
-
- Caffrey M. Crystallizing Membrane Proteins for Structure Determination: Use of Lipidic Mesophases. Ann Rev Biophys. 2009;38:29–51. - PubMed
-
- Klug CS, Feix JB. Biophysical Tools for Biologists: Vol 1 in Vitro Techniques. Elsevier Academic Press Inc; San Diego: 2008. Methods and applications of site-directed spin Labeling EPR Spectroscopy; pp. 617–658. - PubMed
-
- Langen R, Oh KJ, Cascio D, Hubbell WL. Crystal structures of spin labeled T4 lysozyme mutants: Implications for the interpretation of EPR spectra in terms of structure. Biochemistry. 2000;39:8396–8405. - PubMed
-
- Melo M, Ferre R, Castanho M. Antimicrobial peptides: linking partition, activity and high membrane-bound concentrations. Nat Rev Microbiol. 2009;7:245–250. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

