Keeping the LINC: the importance of nucleocytoskeletal coupling in intracellular force transmission and cellular function
- PMID: 22103516
- PMCID: PMC4589539
- DOI: 10.1042/BST20110686
Keeping the LINC: the importance of nucleocytoskeletal coupling in intracellular force transmission and cellular function
Abstract
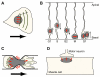
Providing a stable physical connection between the nucleus and the cytoskeleton is essential for a wide range of cellular functions and it could also participate in mechanosensing by transmitting intra- and extra-cellular mechanical stimuli via the cytoskeleton to the nucleus. Nesprins and SUN proteins, located at the nuclear envelope, form the LINC (linker of nucleoskeleton and cytoskeleton) complex that connects the nucleus to the cytoskeleton; underlying nuclear lamins contribute to anchoring LINC complex components at the nuclear envelope. Disruption of the LINC complex or loss of lamins can result in disturbed perinuclear actin and intermediate filament networks and causes severe functional defects, including impaired nuclear positioning, cell polarization and cell motility. Recent studies have identified the LINC complex as the major force-transmitting element at the nuclear envelope and suggest that many of the aforementioned defects can be attributed to disturbed force transmission between the nucleus and the cytoskeleton. Thus mutations in nesprins, SUN proteins or lamins, which have been linked to muscular dystrophies and cardiomyopathies, may weaken or completely eliminate LINC complex function at the nuclear envelope and result in impaired intracellular force transmission, thereby disrupting critical cellular functions.
Figures

Similar articles
-
The interaction between nesprins and sun proteins at the nuclear envelope is critical for force transmission between the nucleus and cytoskeleton.J Biol Chem. 2011 Jul 29;286(30):26743-53. doi: 10.1074/jbc.M111.233700. Epub 2011 Jun 7. J Biol Chem. 2011. PMID: 21652697 Free PMC article.
-
LINC complexes in health and disease.Nucleus. 2010 Jan-Feb;1(1):40-52. doi: 10.4161/nucl.1.1.10530. Nucleus. 2010. PMID: 21327104 Free PMC article. Review.
-
Nuclear lamin isoforms differentially contribute to LINC complex-dependent nucleocytoskeletal coupling and whole-cell mechanics.Proc Natl Acad Sci U S A. 2022 Apr 26;119(17):e2121816119. doi: 10.1073/pnas.2121816119. Epub 2022 Apr 19. Proc Natl Acad Sci U S A. 2022. PMID: 35439057 Free PMC article.
-
The LINC complex is required for endothelial cell adhesion and adaptation to shear stress and cyclic stretch.Mol Biol Cell. 2021 Aug 19;32(18):1654-1663. doi: 10.1091/mbc.E20-11-0698. Epub 2021 Jun 30. Mol Biol Cell. 2021. PMID: 34191529 Free PMC article.
-
Dynamic regulation of LINC complex composition and function across tissues and contexts.FEBS Lett. 2023 Nov;597(22):2823-2832. doi: 10.1002/1873-3468.14757. Epub 2023 Oct 24. FEBS Lett. 2023. PMID: 37846646 Review.
Cited by
-
A historical to present-day account of efforts to answer the question: "what puts the brakes on mammalian hair cell regeneration?".Hear Res. 2013 Mar;297:52-67. doi: 10.1016/j.heares.2013.01.005. Epub 2013 Jan 17. Hear Res. 2013. PMID: 23333259 Free PMC article. Review.
-
Nesprins and Lamins in Health and Diseases of Cardiac and Skeletal Muscles.Front Physiol. 2018 Sep 7;9:1277. doi: 10.3389/fphys.2018.01277. eCollection 2018. Front Physiol. 2018. PMID: 30245638 Free PMC article. Review.
-
FMN2 Makes Perinuclear Actin to Protect Nuclei during Confined Migration and Promote Metastasis.Cell. 2016 Dec 1;167(6):1571-1585.e18. doi: 10.1016/j.cell.2016.10.023. Epub 2016 Nov 10. Cell. 2016. Retraction in: Cell. 2018 Apr 5;173(2):529. doi: 10.1016/j.cell.2018.03.058. PMID: 27839864 Free PMC article. Retracted.
-
Physical biology in cancer. 5. The rocky road of metastasis: the role of cytoskeletal mechanics in cell migratory response to 3D matrix topography.Am J Physiol Cell Physiol. 2014 Jan 15;306(2):C110-20. doi: 10.1152/ajpcell.00283.2013. Epub 2013 Nov 6. Am J Physiol Cell Physiol. 2014. PMID: 24196535 Free PMC article. Review.
-
Structural insights into SUN-KASH complexes across the nuclear envelope.Cell Res. 2012 Oct;22(10):1440-52. doi: 10.1038/cr.2012.126. Epub 2012 Sep 4. Cell Res. 2012. PMID: 22945352 Free PMC article.
References
-
- Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol. 2009;10(1):75–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

