Three-component coupling sequence for the regiospecific synthesis of substituted pyridines
- PMID: 22103772
- PMCID: PMC3262091
- DOI: 10.1021/ja2105703
Three-component coupling sequence for the regiospecific synthesis of substituted pyridines
Abstract
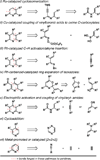
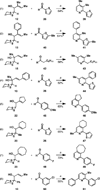
A de novo synthesis of substituted pyridines is described that proceeds through nucleophilic addition of a dithiane anion to an α,β-unsaturated carbonyl followed by metallacycle-mediated union of the resulting allylic alcohol with preformed trimethylsilane-imines (generated in situ by the low-temperature reaction of lithium hexamethyldisilazide with an aldehyde) and Ag(I)- or Hg(II)-mediated ring closure. The process is useful for the convergent assembly of di- through penta-substituted pyridines with complete regiochemical control.
© 2011 American Chemical Society
Figures







Similar articles
-
Stereoselective Conjugate Addition of the Lithium Anion of N-Allyl Imine to Unsaturated Esters: Application to the Enantiospecific Total Synthesis of (-)-Epibatidine.J Org Chem. 2019 Aug 2;84(15):9648-9660. doi: 10.1021/acs.joc.9b01340. Epub 2019 Jul 24. J Org Chem. 2019. PMID: 31293165
-
Synthesis of dihydropyridines and pyridines from imines and alkynes via C-H activation.J Am Chem Soc. 2008 Mar 19;130(11):3645-51. doi: 10.1021/ja7104784. Epub 2008 Feb 27. J Am Chem Soc. 2008. PMID: 18302381 Free PMC article.
-
Synthesis of 2-substituted pyridines via a regiospecific alkylation, alkynylation, and arylation of pyridine N-oxides.Org Lett. 2007 Mar 29;9(7):1335-7. doi: 10.1021/ol070184n. Epub 2007 Mar 1. Org Lett. 2007. PMID: 17328555
-
Isothiourea-mediated one-pot synthesis of functionalized pyridines.Angew Chem Int Ed Engl. 2013 Oct 25;52(44):11642-6. doi: 10.1002/anie.201306786. Epub 2013 Sep 17. Angew Chem Int Ed Engl. 2013. PMID: 24106063 Free PMC article.
-
In search of oligo(2-thienyl)-substituted pyridine derivatives: a modular approach to di-, tri- and tetra(2-thienyl)pyridines.Chemistry. 2011 Oct 10;17(42):11838-43. doi: 10.1002/chem.201101739. Epub 2011 Sep 6. Chemistry. 2011. PMID: 21898622
Cited by
-
Reaction design, discovery, and development as a foundation to function-oriented synthesis.Acc Chem Res. 2015 Mar 17;48(3):663-73. doi: 10.1021/ar500408e. Epub 2015 Feb 10. Acc Chem Res. 2015. PMID: 25668752 Free PMC article.
-
Rh(III)-catalyzed decarboxylative coupling of acrylic acids with unsaturated oxime esters: carboxylic acids serve as traceless activators.J Am Chem Soc. 2014 Feb 19;136(7):2735-8. doi: 10.1021/ja412444d. Epub 2014 Feb 10. J Am Chem Soc. 2014. PMID: 24512241 Free PMC article.
-
Modern applications of low-valent early transition metals in synthesis and catalysis.Nat Rev Chem. 2019 Jan;3(1):15-34. doi: 10.1038/s41570-018-0059-x. Epub 2018 Nov 30. Nat Rev Chem. 2019. PMID: 30989127 Free PMC article.
-
Unified Total Synthesis of the Limonoid Alkaloids: Strategies for the De Novo Synthesis of Highly Substituted Pyridine Scaffolds.Chem. 2022 Oct 13;8(10):2856-2887. doi: 10.1016/j.chempr.2022.09.012. Epub 2022 Oct 4. Chem. 2022. PMID: 37396824 Free PMC article.
-
Photocatalytic Reductive Radical-Polar Crossover for a Base-Free Corey-Seebach Reaction.Chemistry. 2020 Oct 9;26(57):12945-12950. doi: 10.1002/chem.202003000. Epub 2020 Sep 17. Chemistry. 2020. PMID: 32686166 Free PMC article.
References
-
-
For a recent review, see: Henry GD. Tetrahedron. 2004;60:6043–6061.
-
-
-
Name Reactions in Heterocycle Synthesis; Li JJ, Corey EJ, editors. New York: Wiley; 2005. p. 558.
-
-
- Movassaghi M, Hill MD. J. Am. Chem. Soc. 2006;128:4592–4593. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

